Publications
Biopolym. Cell. 2018, 34 (1), 59-71
DOI: 10.7124/bc.000971

J. Org. Chem. 2018, 83 (12), 6275-6289
DOI: 10.1021/acs.joc.8b00077

Org. Biomol. Chem. 2018, 16 (44), 8559-8564
DOI: 10.1039/C8OB02428F
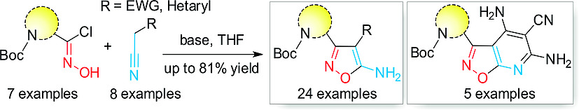
Heterocycl. Commun. 2018, 24 (4), 177-181
DOI: 10.1515/hc-2017-0143
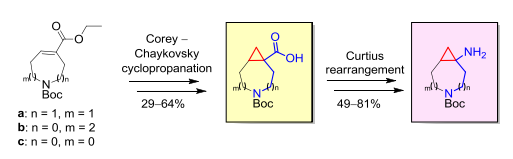
Tetrahedron Lett. 2018, 59 (52), 4611-4615
DOI: 10.1016/j.tetlet.2018.11.047
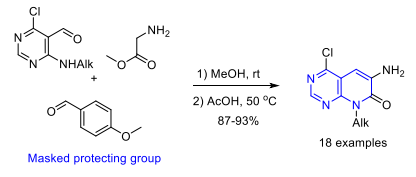
Eur. J. Org. Chem. 2018, 46, 6519-6523
DOI: 10.1002/ejoc.201801204

Eur. J. Org. Chem. 2018, 40, 5585-5595
DOI: 10.1002/ejoc.201800753
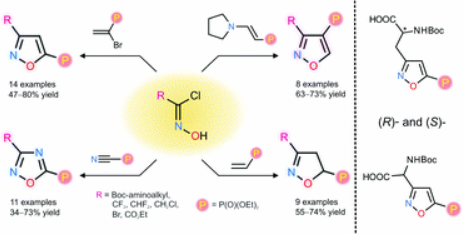
Org. Biomol. Chem. 2018, 16 (47), 9152-9164
DOI: 10.1039/C8OB02257G

Eur. J. Org. Chem. 2018, 47, 6682-6692
DOI: 10.1002/ejoc.201801270

Synthesis 2019, 51 (4), 848-858
DOI: 10.1055/s-0037-1611277
Angew. Chem. 2018, 57 (50), 16442-16446
DOI: 10.1002/anie.201810460

ACS Comb. Sci. 2018, 20 (11), 672-680
DOI: 10.1021/acscombsci.8b00130

J. Org. Chem. 2018, 83 (23), 14350–14361
DOI: 10.1021/acs.joc.8b02071

ACS Comb. Sci. 2018, 20 (7), 461-466
DOI: 10.1021/acscombsci.8b00060

Chem. Heterocycl. Compd. 2018, 54 (8), 789‑795
DOI: 10.1007/s10593-018-2350-7
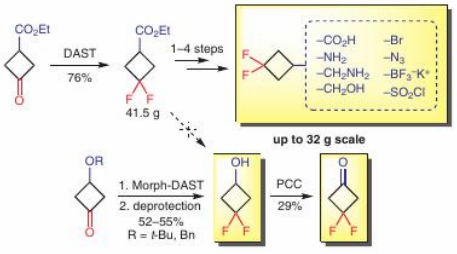
Synthesis 2018, 50 (24), 4949-4957
DOI: 10.1055/s-0037-1610237
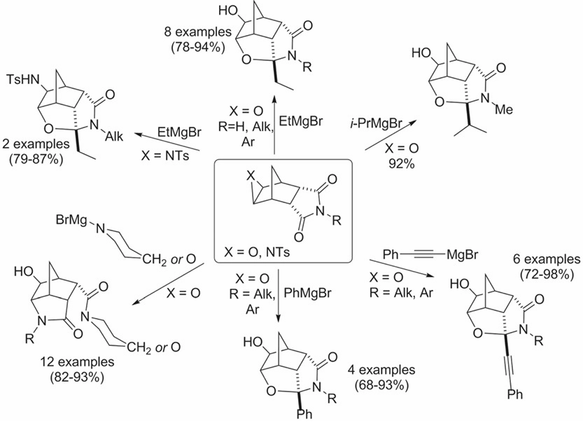
J. Heterocycl. Chem. 2018, 55 (10), 2381-2391
DOI: 10.1002/jhet.3302
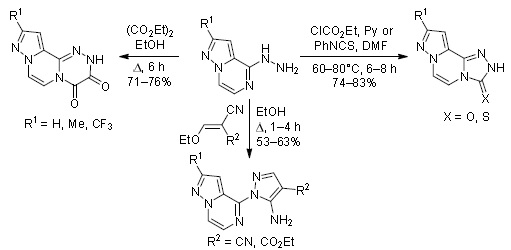
Chem. Heterocycl. Compd. 2018, 54 (7), 710‑716
DOI: 10.1007/s10593-018-2337-4
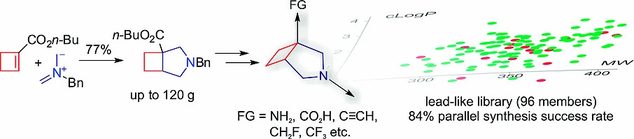
Eur. J. Org. Chem. 2018, 40, 5596-5604
DOI: 10.1002/ejoc.201800972

Synthesis 2018, 50 (18), 3696-3707
DOI: 10.1055/s-0037-1610195
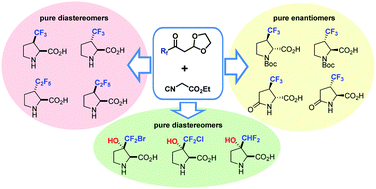
Chem. Commun. 2018, 54 (69), 9683-9686
DOI: 10.1039/c8cc05912h
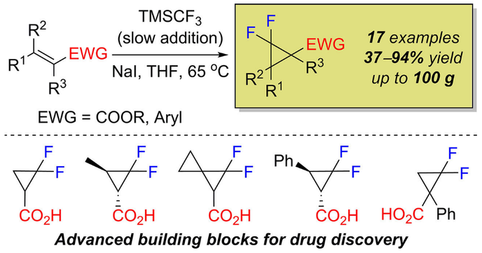
Adv. Synth. Catal. 2018, 360 (21), 4104-4114
DOI: 10.1002/adsc.201801006
Heterocycl. Commun. , 2018, 24 (4), 237-240
DOI: 10.1515/hc-2018-0013
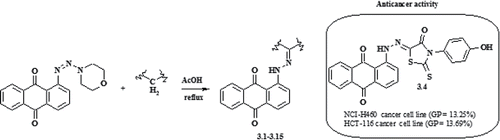
Phosphorus, Sulfur Silicon Relat. Elem. 2018, 193 (7), 409-414
DOI: 10.1080/10426507.2018.1452236
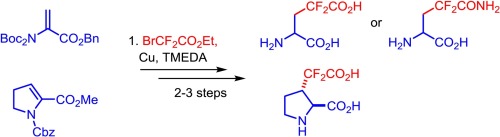
J. Fluorine Chem. 2018, 211, 100-108
DOI: 10.1016/j.jfluchem.2018.03.014
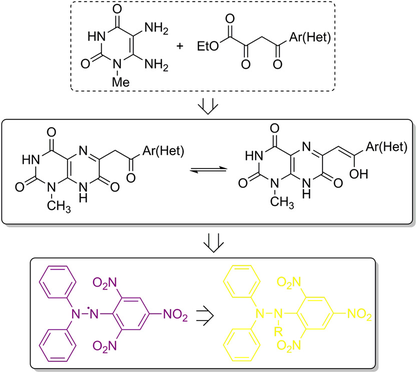
J. Heterocycl. Chem. 2018, 55 (4), 1033-1041
DOI: 10.1002/jhet.3135

Eur. J. Org. Chem. 2018, 27-28, 3826-3833
DOI: 10.1002/ejoc.201800473
Eur. J. Org. Chem. 2018, 22, 2753-2761
DOI: 10.1002/ejoc.201800311

New J. Chem. 2018, 42 (11), 8355-8365
DOI: 10.1039/C7NJ05015A
Tetrahedron 2018, 74 (13), 1355-1421
DOI: 10.1016/j.tet.2018.01.033
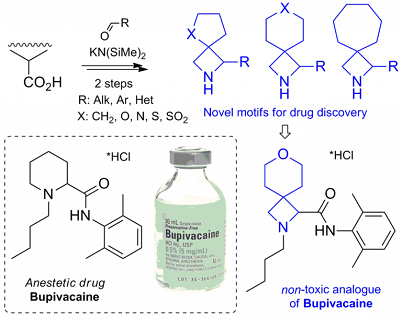
Chem. Eur. J. 2018, 24 (21), 5444-5449
DOI: 10.1002/chem.201800193
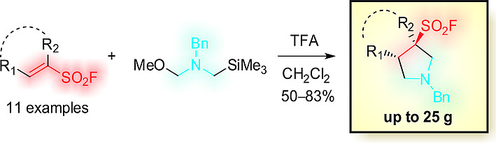
Eur. J. Org. Chem. 2018, (22), 2870-2876
DOI: 10.1002/ejoc.201800521
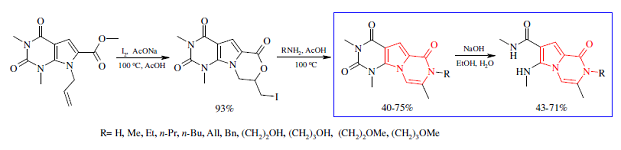
Tetrahedron Lett. 2018, 59 (5), 442-444
DOI: 10.1016/j.tetlet.2017.12.065

RSC Adv. 2018, 8 (10), 5114-5118
DOI: 10.1039/C7RA13141K
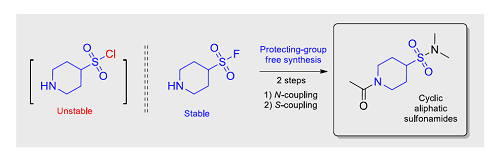
Chem. Eur. J. 2018, 24 (33), 8343-8349
DOI: 10.1002/chem.201801140
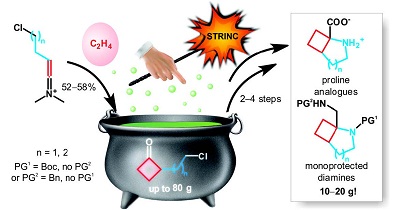
Synthesis 2018, 50 (10), 1973-1978
DOI: 10.1055/s-0037-1609434

Chem. Heterocycl. Compd. 2018, 53 (11), 1242-1247
DOI: 10.1016/j.tetlet.2016.01.094
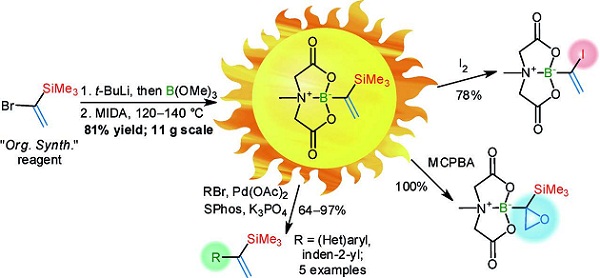
Synthesis 2018, 50 (9), 1857-1861
DOI: 10.1055/s-0036-1591926
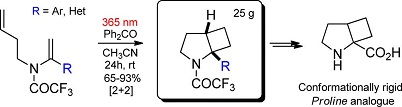
J. Org. Chem. 2018, 83 (3), 1394–1401
DOI: 10.1021/acs.joc.7b02910
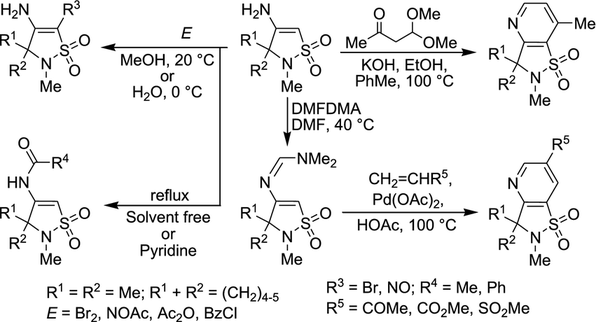
Mol. Divers. 2018, 22 (4), 919–927
DOI: 10.1007/s11030-018-9848-x
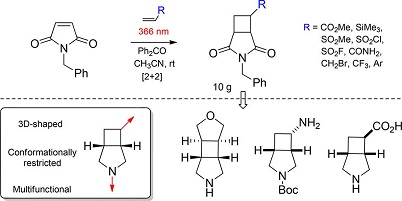
J. Org. Chem. 2018, 83 (12), 6275-6289
DOI: 10.1021/acs.joc.8b00077
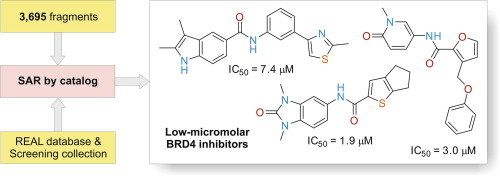
Bioorg. Med. Chem. 2018, 26 (12), 3399-3405
DOI: 10.1016/j.bmc.2018.05.010
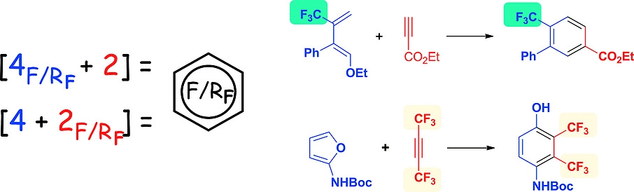
Eur. J. Org. Chem. 2018, (27-28)
DOI: 10.1002/ejoc.201800327
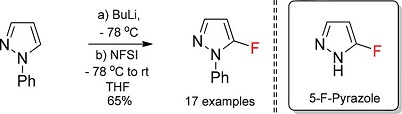
J. Org. Chem. 2018, 83 (6), 3265-3274
DOI: 10.1021/acs.joc.8b00199
Heterocycl. Commun. 2018, 24 (1), 11-17
DOI: 10.1515/hc-2017-0235
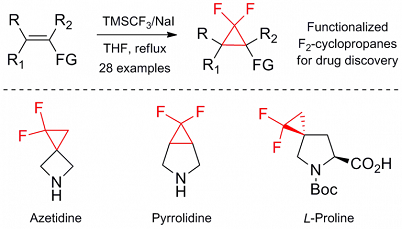
Chem. Eur. J. , 2018, 24 (47)
DOI: 10.1002/chem.201705708
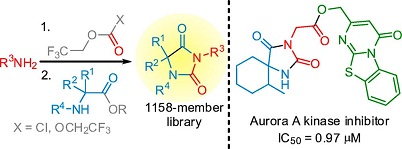
ACS Comb. Sci. 2018, 20 (1), 35-43
DOI: 10.1021/acscombsci.7b00163
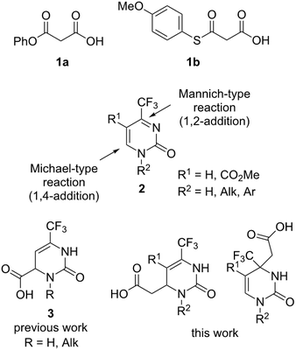
Beilstein J. Org. Chem. 2017, 13, 2617-2625
DOI: 10.3762/bjoc.13.259
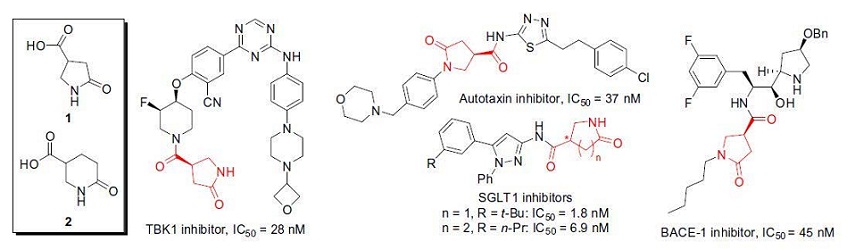
Tetrahedron 2017, 28 (12), 1817-1822
DOI: 10.1016/j.tetasy.2017.10.027
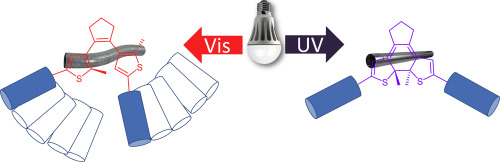
Biochim Biophys Acta Biomembr 2017, 1859 (12), 2505-2515
DOI: 10.1016/j.bbamem.2017.09.021
