C-H Activation Catalysts
C-H activation catalysts are innovate organometallic complexes and organic compounds that enable the direct functionalization of carbon-hydrogen bonds, transforming traditionally inert C-H bonds into reactive sites. Their remarkable ability to modify organic molecules with high precision has significant applications in advanced organic chemistry.
Download SD files
Chiral Catalysts
Chiral catalysts are specialized molecules or complexes that work as enantioselective catalysts and facilitate asymmetric chemical transformations preferring one of the stereoisomers. These catalysts, including metal-containing complexes with chiral ligands and organic molecules with defined stereochemistry, revolutionized the story of asymmetric synthesis. They provide highly efficient routes to optically pure compounds, which are essential for pharmaceutical development and the production of precise chemicals.
Download SD files
Cross-Coupling Catalysts
Cross-coupling catalysts, including Buchwald catalysts and precatalysts, are essential organometallic compounds in organic synthesis, enabling the formation of complex molecular structures with high precision through carbon-carbon, carbon-oxygen, and carbon-nitrogen bond formation and others. These catalysts have significantly advanced synthetic chemistry in pharmaceuticals, materials science, and advanced chemical manufacturing.
Buchwald Precatalysts
Buchwald precatalysts are advanced organometallic complexes, primarily based on palladium, designed by Prof. S. Buchwald to facilitate efficient cross-coupling reactions in organic synthesis. These precatalysts are distinguished by their ability to generate active catalytic species in situ, offering enhanced stability, storage, and handling compared to traditional catalysts.
Download SD files
Other Cross-Coupling Catalysts
Cross-coupling catalysts, including complexes based on nickel (alternative palladium) or other metals, demonstrate varied reactivity, selectivity, and efficiency. They offer diverse capabilities in organic synthesis, enabling the formation of unique C-C, C-N, C-O, and C-S bonds under a wide range of reaction conditions.
Download SD files
Hydrogenation Catalysts
Hydrogenation catalysts are modified transition metals or their compounds, such as ruthenium, palladium or nickel metalocomplexes, that facilitate the addition of hydrogen to unsaturated organic compounds, enabling key transformations in chemical synthesis.
Download SD files
Inorganic Catalysts
Inorganic catalysts are powerful materials composed of non-carbon elements that dramatically accelerate chemical reactions while remaining unchanged. These catalysts play a critical role in optimizing industrial processes—from petrochemicals to environmental applications—by enhancing reaction rates and cutting energy costs. With unmatched stability and efficiency, inorganic catalysts are key to driving innovation and sustainability in industries worldwide.
Download SD files
Low Energy Photoredox Catalysts
Low-energy photoredox catalysts include iridium- and osmium-based complexes that exhibit enhanced photosensitization in the longer-wavelength region compared to conventional photocatalysts. Iridium catalysts are highly effective in sp²-sp³ coupling, decarboxylative arylation and other photoredox transformations, offering versatile reactivity under mild conditions. Osmium catalysts, on the other hand, serve as powerful sensitizing agents in biochemical and medical research, enabling precise photoactivation in biological systems. Additionally, this category features two biotin-conjugated molecules designed for use alongside osmium catalysts in targeted studies, further expanding their applications in bioanalytical research.
Download SD files
Metathesis Catalysts
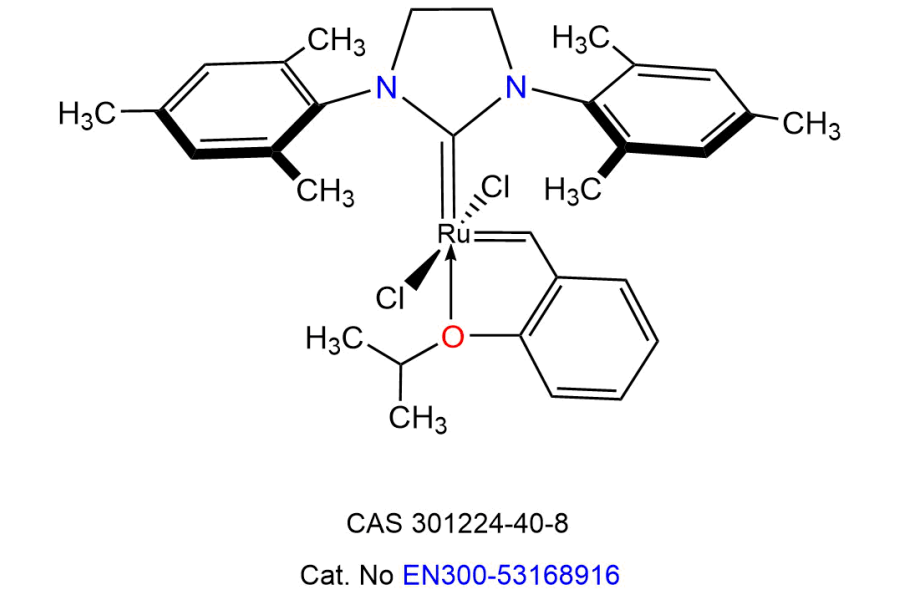
Metathesis catalysts, particularly ruthenium-based complexes such as Grubbs and Hoveyda-Grubbs catalysts, represent a groundbreaking advancement in organic synthesis by enabling the redistribution of carbon-carbon double bonds. These exceptional catalysts function via a unique mechanism involving metal-carbene intermediates, facilitating a wide range of transformations, including ring-closing metathesis (RCM), cross-metathesis (CM), and ring-opening metathesis polymerization (ROMP).
Download SD files
N-Heterocyclic Carbene (NHC) Complexes
N-Heterocyclic Carbene (NHC) complexes are advanced chemical compounds featuring a central carbene unit, which is bonded to a heterocyclic ring structure. Known for their exceptional stability and reactivity,facilitating a wide range of catalytic reactions. These complexes are widely utilized in organic synthesis, pharmaceuticals, and materials science, enabling more efficient reactions with minimal side products and improved selectivity.
Download SD files
Organocatalysts
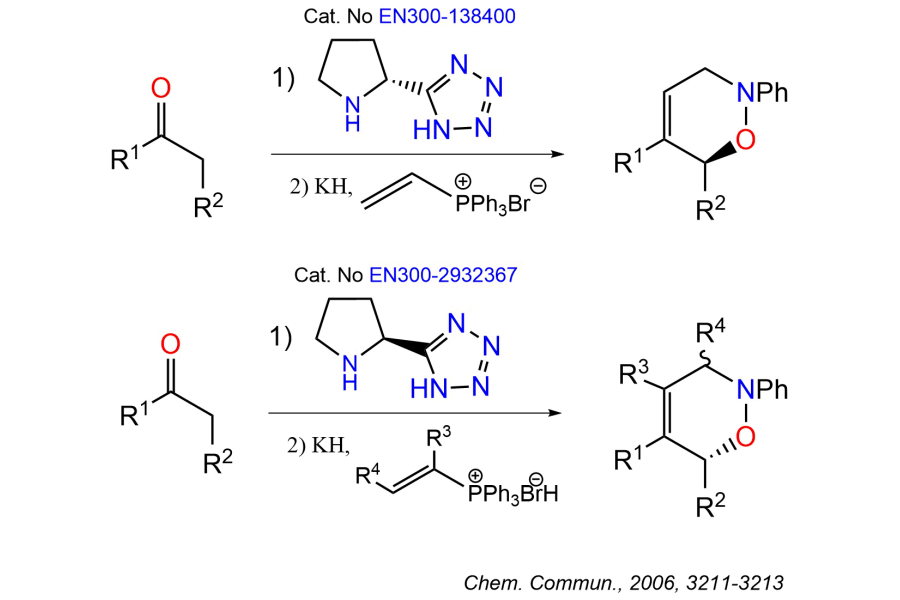
Organocatalysts are small organic molecules that effectively catalyze chemical reactions without requiring metal centers, offering unique advantages in asymmetric synthesis and green chemistry. These catalysts, including various organonitrogen, organophosphorus, and other compounds, enable stereoselective transformations with high efficiency and minimal environmental impact.
Download SD files
Photoredox Catalysts
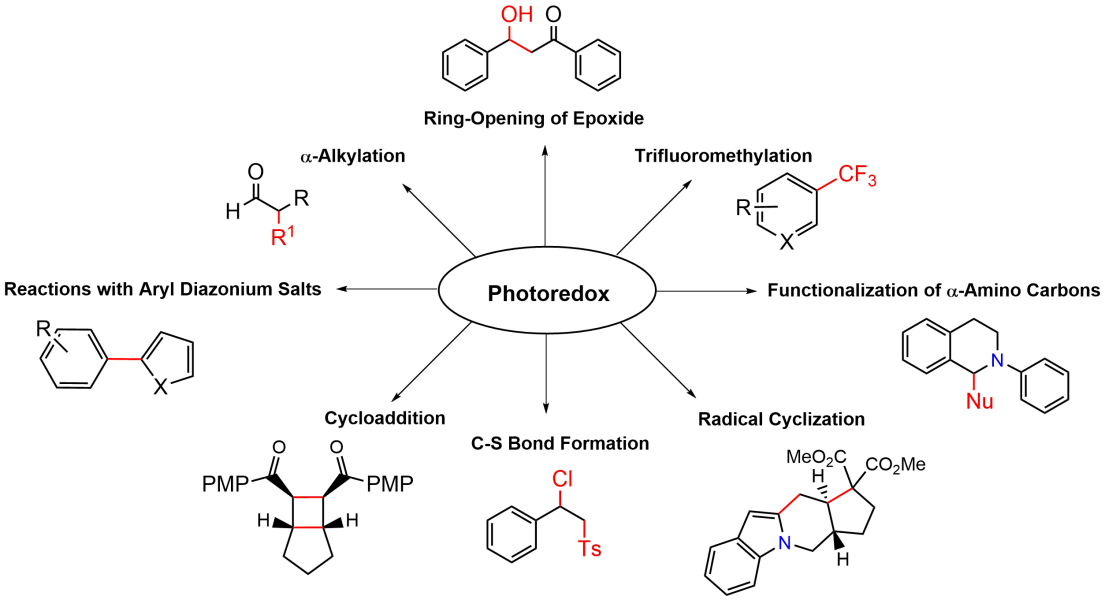
Photocatalysts are specialized compounds that harness light energy to facilitate chemical transformations. These catalysts include a wide range of organic molecules and metal complexes, such as those containing ruthenium(II) and iridium(III). They enable unique reaction pathways through single-electron transfer (SET) processes and the generation of reactive intermediates under mild conditions, making them particularly valuable in environmentally friendly chemistry.
Download SD files
Transition Metal Catalysts
Transition metal catalysts, including complexes of copper, iron, cobalt, and other metals, are essential for a wide range of chemical transformations in both industrial and laboratory settings. They are especially important in hydroformylation reactions, oxidation processes, and polymerization, with broad applications in pharmaceutical synthesis, materials science, and large-scale chemical production.
Buchwald Ligands
Buchwald ligands are sterically hindered and electron-rich phosphine compounds belonging to the class of dialkylbiarylphosphines. They are highly effective in various palladium-catalyzed cross-coupling reactions and enable exceptional catalytic activity and stability under mild reaction conditions, particularly in challenging C-N, C-O, and other bond formations. The systematic modification of their biaryl backbone and phosphine substituents allows fine-tuning of their electronic and steric properties, making them valuable tools for process and method development in academic and industrial settings.
Download SD files
Cross-Coupling Ligands
Specialized organic molecules that facilitate metal-catalyzed coupling reactions. By binding to metal centers, these ligands enhance catalyst stability and regulate reaction selectivity. Common examples include phosphines and N-heterocyclic carbenes, which are indispensable in Suzuki, Buchwald-Hartwig, and other cross-coupling reactions widely employed in pharmaceutical development and materials synthesis
Download SD files
Ligands for Asymmetric Catalysis
Ligands for asymmetric catalysis include both chiral and non-chiral molecules that create specific three-dimensional environments around metal centers, facilitating the synthesis of enantiomerically pure compounds. These ligands are essential in asymmetric catalysis as they influence the spatial arrangement of reactants, guiding the course of enantioselective reactions and promoting the preferential formation of the desired stereoisomers.
Download SD files
Ligands for C-H Activation
Molecules that facilitate the selective functionalization of traditionally unreactive carbon-hydrogen bonds, by stabilizing metal catalysts and directing their activity toward specific C-H bonds. These ligands enable precise control over pathways of the C-H bond functionalization. Their distinctive electronic and steric properties render them indispensable tools in contemporary organic synthesis.
Download SD files
Ligands for Ullmann Сross-Сoupling
Ligands for Ullmann cross-coupling are organic molecules that facilitate C(sp²)-N and C(sp²)-O bond formation under copper catalysis at ambient temperature. These ligands offer a cost-effective alternative to conventional phosphine ligands and palladium catalysts commonly used in Buchwald-Hartwig cross-coupling reactions. By stabilizing copper intermediates and enhancing catalytic efficiency, they enable milder reaction conditions while maintaining high yields and selectivity. Their applications extend across various industries, including pharmaceuticals, agrochemicals, and advanced materials, where selective heteroatom coupling plays a critical role in molecular design.
Download SD files
N-Heterocyclic Carbene (NHC) Ligands
Cyclic compounds characterized by a divalent carbon atom situated between two nitrogen atoms, enabling the formation of exceptionally strong bonds with metal centers. These molecules exhibit superior thermal stability and distinctive electronic properties compared to phosphine ligands, making them indispensable in cross-coupling reactions, olefin metathesis, and a wide range of transformations in organic synthesis. Their modular design allows for precise tuning of steric and electronic properties through structural modifications, further enhancing their versatility.
Download SD files
Other Phosphine Ligands
Phosphine ligands are organophosphorus compounds that play an important role in transition metal catalysis by modulating the electronic and steric properties of metal centers. These versatile ligands form stable metal-phosphorus bonds via their lone pair of electrons, enabling precise control over catalyst reactivity and selectivity. Their tunable nature, achieved through variations in substituents on the phosphorus atom, makes them indispensable tools in modern organic synthesis and catalytic processes.
Download SD files
Photoredox Ligands
Light-sensitive molecules that facilitate electron transfer in catalytic processes. They absorb visible light to form excited states, enabling single-electron transfer (SET) pathways. This allows for mild and selective organic transformations. Photoredox chemistry becomes increasingly relevant, offering environmentally friendly and mild reaction conditions while enabling complex transformations, such as C–C bond formation, oxidative couplings, and radical-mediated processes, that are challenging to achieve using conventional methods.
Download SD files
Ligands for Other Types of Catalysis
Ligands for various types of catalysis, designed to support broad applications in research and industry. They enable enhanced reactivity and selectivity in processes ranging from organic synthesis to materials development. A versatile choice for advancing catalytic performance across diverse chemical transformations.