Library synthesis is crucial in modern drug discovery, enabling rapid exploration of chemical space to identify novel therapeutic candidates. We offer fast and cost-efficient supply of the building block sets from our entire catalog. Select whichever building blocks you like or consider our range of preselected and plated sets. We will deliver the selected building blocks either dry in any custom mg- or µmol amounts or in solutions for the immediate use in library synthesis. We welcome business partnerships that aim at just-in-time, economical access to the large numbers of diverse building blocks, such as Quick Building Blocks (Christopher J. Helal, et al. ACS Medicinal Chemistry Letters 2019 10 (8), 1104-1109).
We’re happy to use any containers you supply – tubes, vials, or reaction blocks. If easier, you can also choose from our extensive selection, including 1- and 2-dram reaction vials with pressure-relief caps.
Please download our stock building block database from here to make your selection.
Pre-plated Building Block sets
Selecting a handful of diverse and meaningful building blocks from tens of thousands of compounds available in stock can be tedious and resource-intensive. We have carefully selected the most representative reagents and plated them at 100 mM in DMSO for quick supply and to minimize the access costs. Most importantly, all compounds were tested by Enamine's parallel chemistry department under widely used reaction conditions to match an over 80% synthesis success rate requirement.
Our pre-plated Building Block sets are designed for fundamental medicinal chemistry reactions, including amide bond formation, Suzuki–Miyaura cross-coupling, azide-alkyne [3+2] cycloaddition, and Mitsunobu reactions.
Most popular formats available for immediate supply
Catalog No.
Carboxylic Acids
CABB-380-Y-50
Compounds
380
1 plate
Amount
50 μL of 100 mM DMSO solutions
Plates and formats
384-well microplates, 384 compounds per plate
Price
Download file
Catalog No.
Amines
ABB-380-X-10
Compounds
380
4 plates
Amount
10 µL of 100 mM DMSO solutions
Plates and formats
96-well plates, 96 compounds per plate
Price
Download file
Catalog No.
Azides
AZD-380-X-100
Compounds
380
4 plates
Amount
100 μL of 100 mM DMSO solutions
Plates and formats
96-well microplates, 96 compounds per plate
Price
Download file
Catalog No.
Alcohols
ALC-380-Y-50
Compounds
380
1 plate
Amount
50 μL of 100 mM DMSO solutions
Plates and formats
96-well microplates, 96 compounds per plate
Price
Download file
Catalog No.
Aryl Boronics
BBB-380-Y-50
Compounds
380
1 plate
Amount
50 μL of 100 mM DMSO solutions
Plates and formats
384-well microplates, 384 compounds per plate
Price
Download file
Catalog No.
Acetylenes
ACET-320-Y-25
Compounds
320
1 plate
Amount
25 µL of 100 mM DMSO solutions
Plates and formats
384-well microplates, 320 compounds per plate
Price
Download file
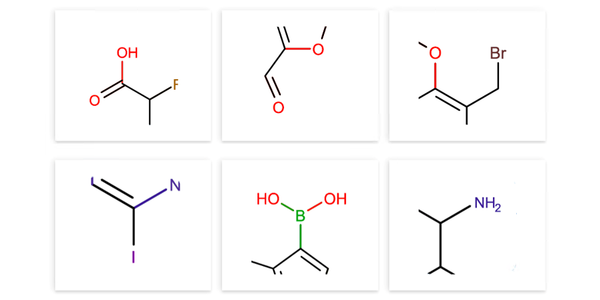
Carboxylic Acids. This set of 384 diverse building blocks has been selected from over 20,000 carboxylic acids demonstrating excellent performance in the amide coupling reactions. Over 95% are cyclic compounds containing one or two rings, covering a broad range of aromatic heterocyclic systems, with 25% of the selection comprising non-aromatic compounds. The set includes compounds with varying degrees of flexibility and spatial arrangements between the cyclic scaffold and the carboxylic acid group. Approximately 25% of the set features the carboxylic acid group directly attached to the cyclic fragment, 33% are separated by one atom, 29% by two atoms, and 8% by longer linkers. Compounds with problematic functionalities, such as ketones, aldehydes, nitro compounds, acyclic derivatives, sulfur in non-cyclic forms, sulfoxides, boron-containing molecules, reactive groups, and salts, were removed.
Amines. The amine sets consist of four diverse collections (96 compounds each): primary and secondary anilines (including heterocyclic) and primary and secondary aliphatic amines that were selected from over 37 000 primary and over 20 000 secondary amines from stock. Only free bases or mono-hydrochlorides were included. All compounds have fewer than 19 heavy atoms and 95% have 0-3 rotatable bonds. Free alcohols were also excluded.
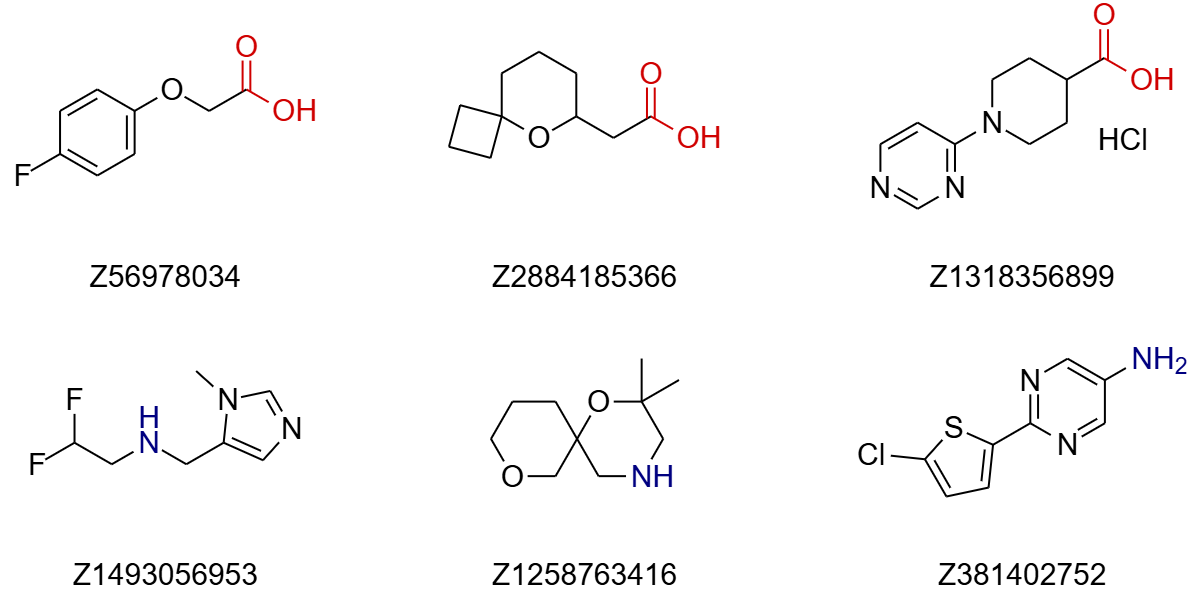
Fig.1 Examples of structures from pre-plated amine and carboxylic acid sets
Aryl Boronic Acid Pinacol Esters. For aryl boronic acid pinacol esters, the selection includes 384 diverse compounds (a separate set of boronic acids is also available, though slightly less diverse). The selection includes aromatic and heteroaromatic boron derivatives, with a wide representation of different heterocycles. Compounds prone to hydrolysis or workup issues, such as halogen-substituted pyridines (e.g., 2- or 4-chloro/fluoro), phenols, esters, aldehydes, ketones, unsubstituted anilines, nitro compounds, and salts, were removed. The MW of introduced fragments (excluding Bpin) ranges from 67 to 233 Daltons, with all fragments containing up to 5 rotatable bonds (80% with 1–2 RotB).
Alcohols. Enamine’s alcohol sets include two separate selections: one for primary and one for secondary alcohols, with 192 compounds in each. Almost all compounds contain at least one ring, although a few acyclic compounds were also included. Molecules with some functional groups were manually excluded, such as nitro compounds, alkynes, azides, boron derivatives, iodine derivatives, polyhalogenated compounds, derivatives of sulfur in lower oxidation states (with few cyclic derivatives included), and compounds containing long chains. Primary alcohols have MWs ranging from 90 to 287 Daltons (75% with MW < 200), and 67% contain 1–2 rotatable bonds (RotB). Secondary alcohols have MWs ranging from 84 to 300 Daltons (70% with MW < 200), and 75% contain 0–2 RotB.
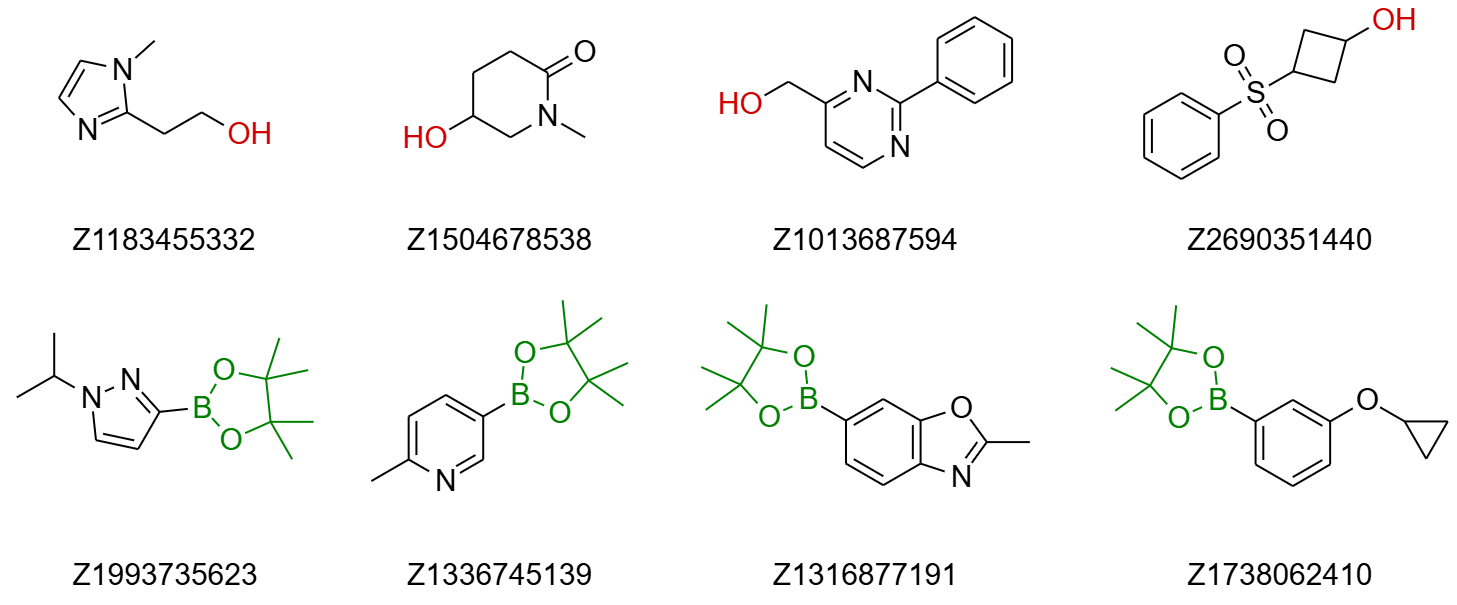
Fig.2 Examples of building blocks for Mitsunobu and Suzuki reactions
Azides. The azide building block set includes 380 diverse compounds optimized for click-chemistry applications, such as azide-alkyne [3+2] cycloaddition. The resulting 1,2,3-triazoles are stable compounds and have been proposed as bioisosteres for amide bonds. The selection comprises both aromatic and aliphatic azides, covering a wide range of functional groups and structural frameworks to ensure broad compatibility. Compounds prone to decomposition, or instability during storage were excluded. Learn more ->
Acetylenes. The acetylene set consists of 320 diverse building blocks tailored for click-reaction chemistry. The selection includes only terminal alkynes, with a focus on compounds that offer high reactivity and stability under standard reaction conditions. Aromatic and aliphatic acetylenes are well-represented, ensuring compatibility with a variety of applications. The MW of compounds in the set ranges from 60 to 300 Daltons, and more than 80% of the compounds contain at least one ring.
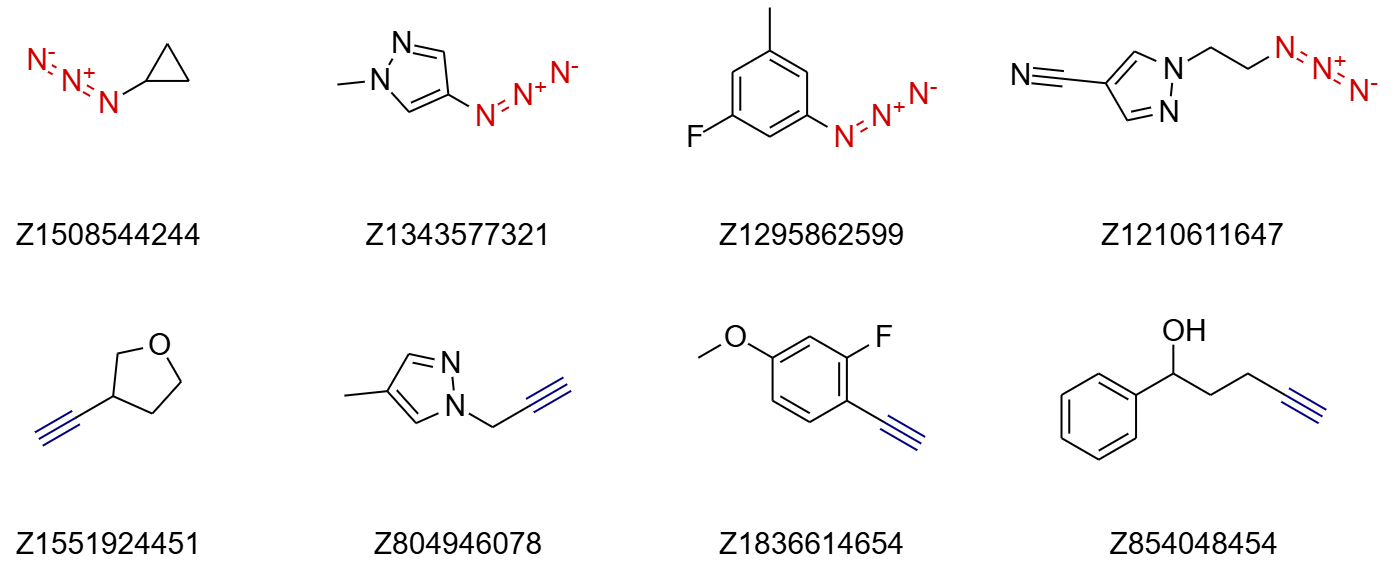
Fig.3 Examples of building blocks from pre-plated azide and acetylene sets