For easy and efficient exploration of novel protein targets and proteomics screening platforms
Photoaffinity labeling (PAL) is a successful and widespread strategy for ligand-binding site mapping, protein target-fishing, and target identification with affinity-based probes (AfBP). Medicinal chemists and structural biologists often use this powerful technique to reveal molecular targets after phenotypic screens and investigate signaling pathways and interactions between proteins of interest. PAL has become one of the most used tools in chemical proteomics and the studies of protein-protein interactions (PPIs).
Diazirines, benzophenones, and aryl azides are traditional, well-described photocrosslinkers (PLs) used in PAL. Upon photoirradiation, these moieties produce highly reactive species that quickly react with adjacent protein parts resulting in covalent binding, which can be further identified by mass-spec analysis and other methods. We at Enamine have been working on synthesizing new PLs containing molecules for years and now offer the largest and most diverse source of Photoaffinity Compounds, including a vast collection of Fully Functionalized Compounds.
Download SD files
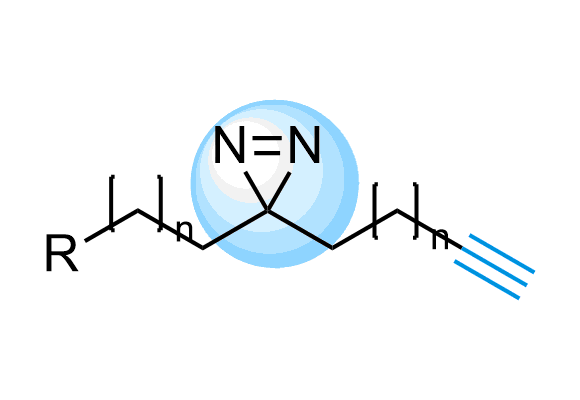
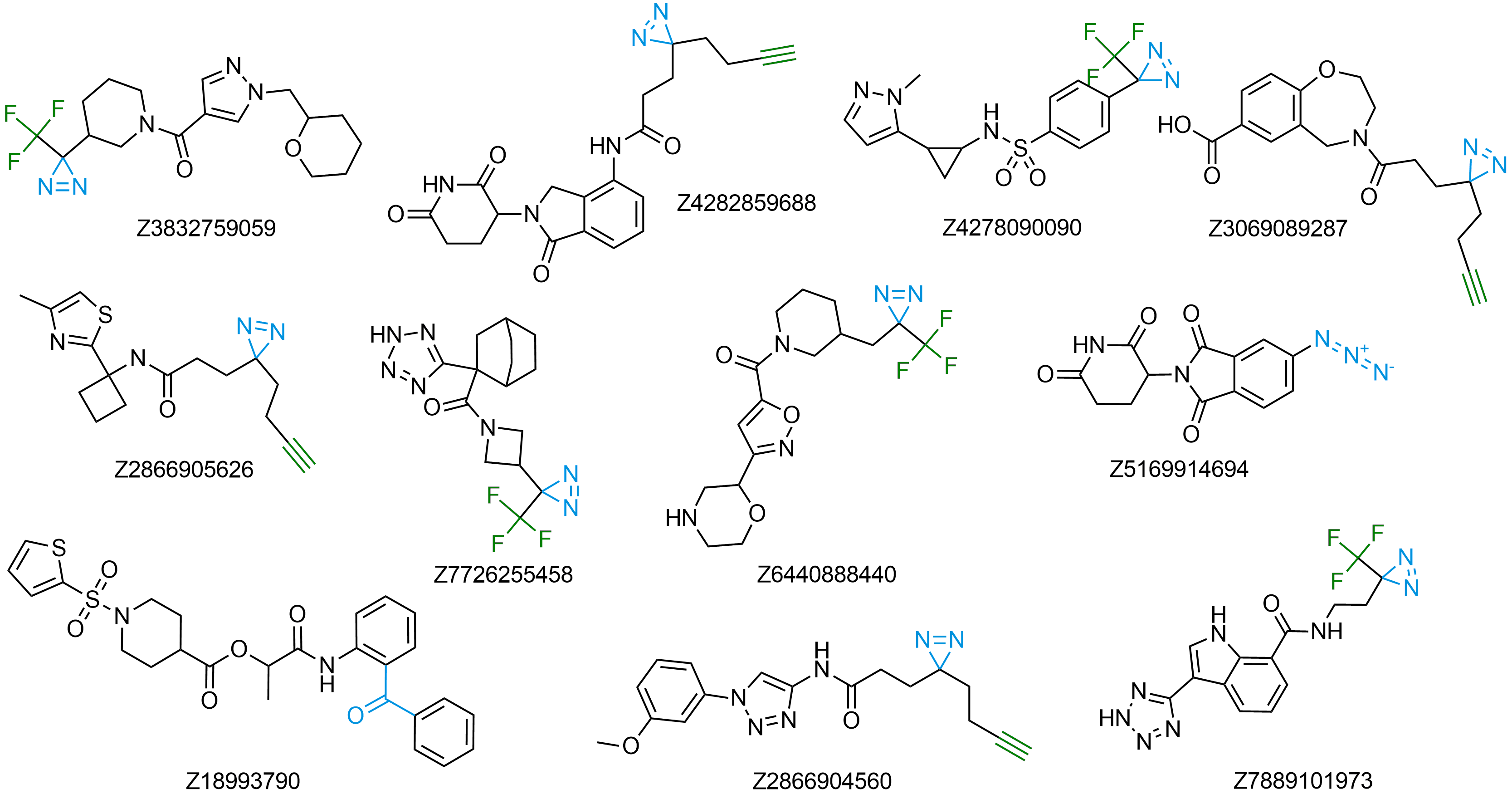
Fully Functionalized Compounds
The use of diazirine in probe molecules, together with the functional acetylene group, allows screening directly in cells. Originally described in the Cell paper2 by Ben Cravatt, this approach has been adopted by other research groups for several successful projects, including ‘direct-to-biology’ high-throughput chemistry screening platform reported by GSK researchers.
We offer over 6 000 Fully Functionalized Compounds from stock and more than 100 million REAL compounds.
Download Fully Functionalized compounds in stock
Download REAL Fully Functionalized Compounds
Browse our building blocks
Trifluoromethyl Diazirines
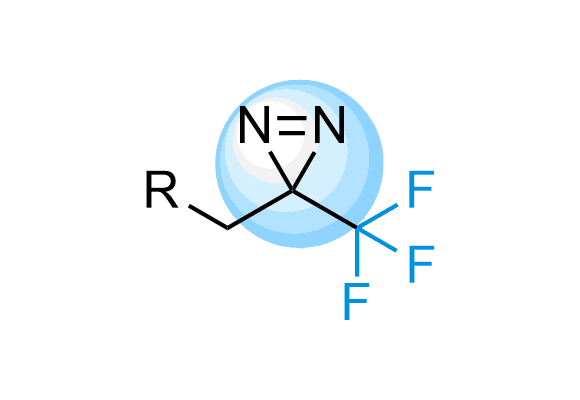
Trifluoromethyl diazirines work more selectively on target because the isomeric diazo compounds formed as side products upon photoirradiation are relatively unreactive and do not largely interfere. We offer over 700 Trifluoromethyl diazirine compounds from stock to select from and more than 300 thousand REAL compounds that we can synthesize on demand using our off-the-shelf CF3-diazirine Building Blocks.
Download ready Trifluoromethyl Diazirine compounds
REAL CF3-diazirine photoaffinity ligands
Benzophenones
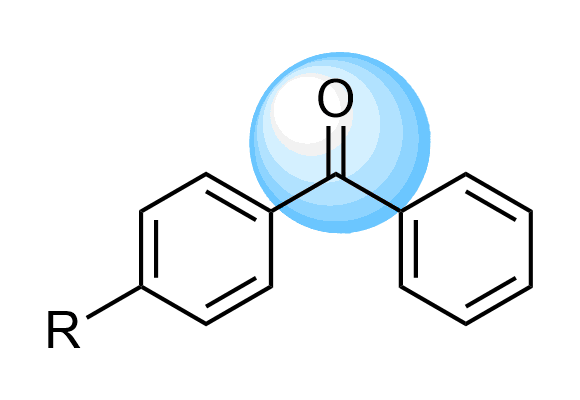
We offer over 5 000 Benzophenone Compounds from stock and will be happy to help you with the design and synthesis of new derivatives.
Aryl Azides
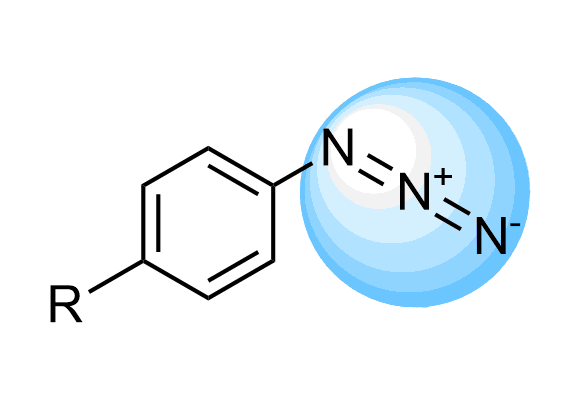
We offer over 50 Arylazides from stock with an extensive array of novel azides that can be synthesized on demand based on our massive collection of azide building blocks.
Synthesis
We have refined our parallel chemistry protocols to efficiently synthesize Photoaffinity Compounds using our own collection of photoaffinity building blocks. This collection is the largest in the world, providing access to all classes of crosslinkers having a wide diversity of functional groups to use in synthesis. Over 15 000 ready-to-use Photoaffinity Compounds have been already synthesized. You can additionally select from over 1 million REAL Compounds bearing photoaffinity warheads. These compounds will be synthesized within just 3-4 weeks with a high (80%) success rate. We also offer synthesis of any bespoke compounds bearing specific photoaffinity moieties attached to selected backbones as well as compound libraries virtually of any size.
Selected publications
-
A direct-to-biology high-throughput chemistry approach to reactive fragment screening.
Thomas R. P. et al. Chem. Sci. 2021, 12, 12098-12106. DOI: 10.1039/D1SC03551G -
Ligand and Target Discovery by Fragment-Based Screening in Human Cells.
Parker C. G. et al. Cell. 2017, 168 (3), 527-541. DOI: 10.1016/j.cell.2016.12.029 -
Fully Functionalized Small-Molecule Probes for Integrated Phenotypic Screening and Target Identification.
Cisar J. S. and Cravatt B. F. J. Am. Chem. Soc. 2012, 134, 25, 10385–10388. DOI: 10.1021/ja304213w