In drug development, great attention is paid to the availability of new pharmaceutical products and their quality. The crucial stages of the drug development process are the identification, quantitation, and control of pharmaceutical impurities. The quantity of unwanted compounds determines the overall safety of the final pharmaceutical product. The restrictions of the impurity content in active pharmaceutical ingredients (APIs) and drug formulations are given in such compendia as USP, EP, BP, JP, and ChP.
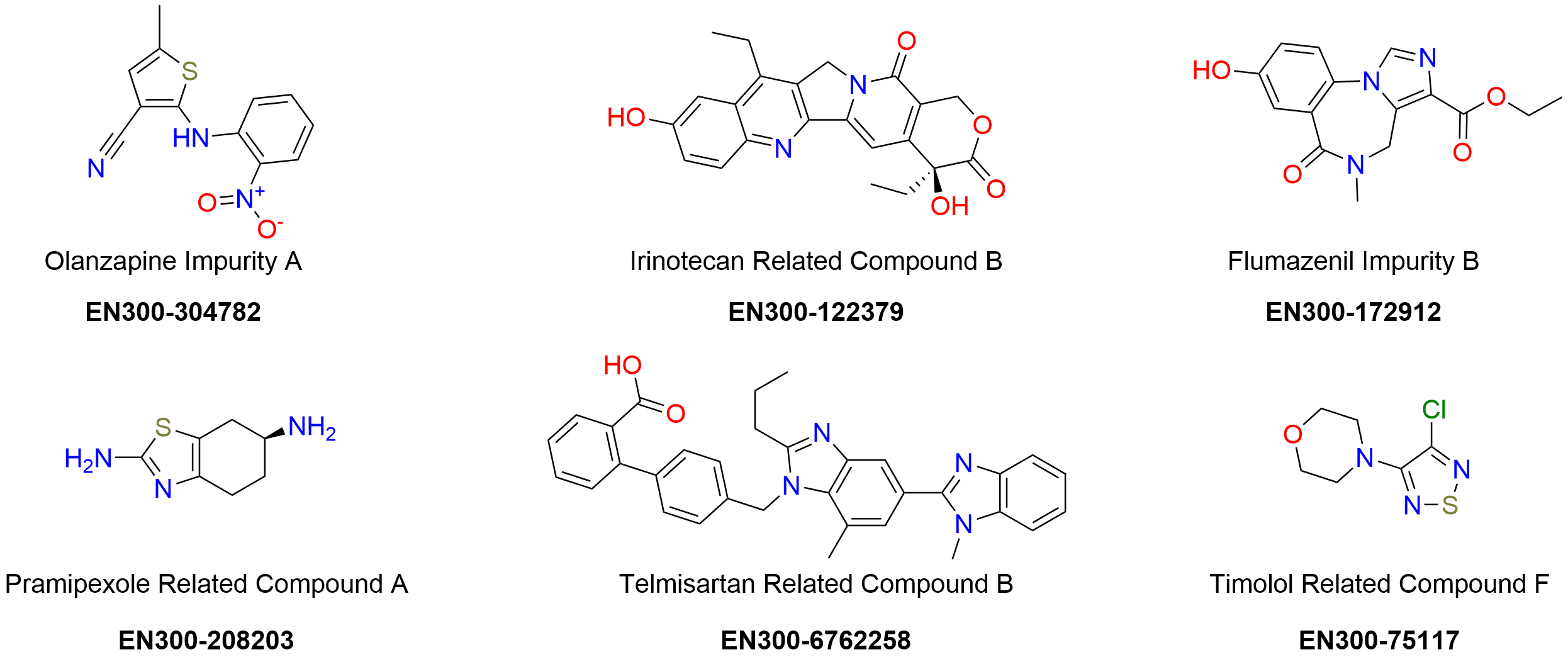
Enamine has precisely documented scientific expertise in organic synthesis and analytical chemistry, which empowers the synthesis and identification of previously unidentified impurities. Our catalog includes 692 drug impurity reference standards in the stock. The certificates of analysis are given for all compounds, they include clear-cut identity and purity data validated using NMR, HPLC/MS, and/or GC/MS methods.
Download
692 compounds
Drug impurity profiling
Enamine provides services in the analysis of API’s to identify impurities. We investigate all steps in the production process assessing any compound or solvent involved in it that could possibly be responsible for the formation of impurities or degradation products. This comprehensive analysis allows a reliable prediction of the structure of the unknown impurities, identification of their generation mechanism, and subsequently strategic impurity management.
Custom Synthesis
Our strong point and competitive advantage are in the design of synthesis routes. We don’t require documented synthesis procedure to produce your compound of interest. In most of the cases, we can propose a realistic synthesis scheme from scratch and successfully realize it producing a desirable amount of compound (from mg to gram scale) in the requested purity.