The largest and most reliable source of novel covalent modifiers
153 430 compounds
Covalent screening has become an integral part of Drug Discovery and is now playing an essential role in hit finding, developing novel screening techniques, identifying new protein targets, and assessing their drugability.
We offer a wide range of covalent compound classes, from acrylamides and chloroacetamides to recently established niche classes whose stability and reactivity are yet to be studied. All compounds have been synthesized at Enamine using our in-house developed synthesis protocols and elaborated purification methods. This has allowed us to enumerate REAL Compound arrays – new covalent compounds that we can confidently synthesize within only 4 weeks.
We will be happy to help you with the covalentization of your scaffold, lead compound or simply give advice on covalent warhead attachment and its type.
Download SD files
Acrylamides
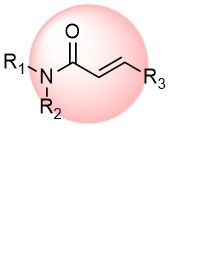
Acrylamides are one of the most popular classes of covalent warheads used for irreversible binding to non-catalytic cysteines of target proteins to enhance pharmacological potency and selectivity. We offer the most diverse collection of the terminal and substituted acrylamides, enabling the discovery of new covalent binders with fine-tuning reactivity and affinity to a target.
Over 42k AcrylamidesChloroacetamides
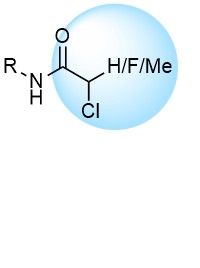
Chloroacetamides are commonly used to develop new covalent probes for challenging targets. Ease of adducts interpretation and high reactivity are their main advantages. Recently synthesized more stable and less reactive 2-chloropropionamides and chlorofluoroacetamides represent a high potential for further discoveries.
Over 15k ChloroacetamidesSulfonyl Fluorides
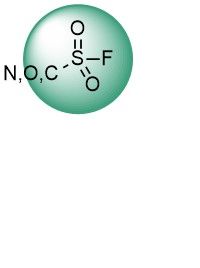
Sulfonyl fluorides have emerged as new, but already well-established, promising covalent binders targeting multiple nucleophilic amino acids such as Tyr, Lys, Ser, His, and Thr. Recent advances, including the application of SuFEx electrophiles in proteomics screens, reveal new horizons for this type of warhead. Their high stability and moderate reactivity allow the discovery of highly selective irreversible binders.
Over 6k Sulfonyl FluoridesBoronics and Formyl Boronates
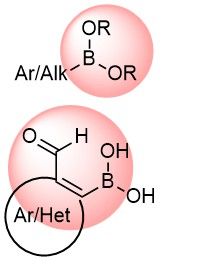
Boronic compounds occupy a special place in medicinal chemistry, including five FDA-approved drugs to date. Boron functional groups are considered covalent binders but are also often utilized in prodrugs, soft drugs, or as various carriers. We focused our efforts on the synthesis of new boronics that can be applied in the discovery of new selective Ser, Thr, and Lys reversible binders.
Over 8k Boronics and Formyl BoronatesEpoxides, Aziridines
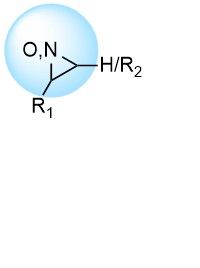
Epoxides and aziridines received relatively little attention, at the same time being among the first developed covalent drugs, including a number of nature-derived antibiotics such as fosfomycin.
Both epoxides and aziridines react irreversibly through SN2 mechanism with the formation of adducts, often with Cys residues.
Vinyl Sulfones
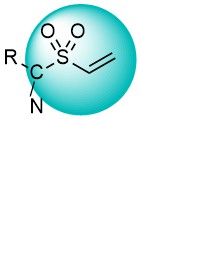
Vinyl sulfones and vinyl sulfonamides are active binders for Lys and Cys residues. They react faster than acrylamides and have shown some preference for binding Lys over Cys. These warheads have been widely used in the development of new selective inhibitors of kinases and other enzymes. An extensive set of versatile vinyl sulfonyl derivatives covering a wide reactivity range is available from Enamine.
Over 9k Vinyl SulfonesCyanoacrylamides
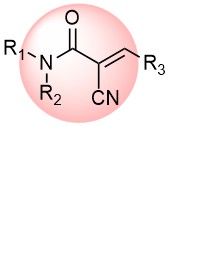
Cyanoacrylamide is a reversible covalent warhead acting on Cys residues. The introduction of a small CN group into α-position of acrylamide dramatically alters its properties, making it a distinct class of covalent binders. Most of them are more active than corresponding acrylamides, but activity can be fine-tuned by appropriate substitution. The presence of the motif in drugs supports its significance in the future of drug discovery.
Over 4k CyanoacrylamidesActivated Acetylenes
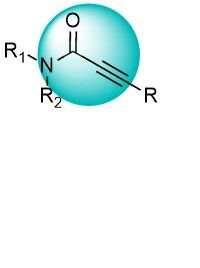
Activated acetylenes can irreversibly bind to cysteine residues. Selectivity, leading to fewer off-target interactions, could be achieved by tuning from acrylamide to propionamide derivatives. These warheads are well-validated and represented by approved drugs. Besides thousands of compounds in stock, we can quickly synthesize unique molecules on demand.
Aldehydes and Salicylic aldehydes
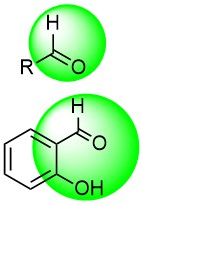
Aldehyde is a common chemical functionality, able to target both catalytic and noncatalytic cysteine residues by forming a reversible covalent bond. Also, they are able to target lysines by the formation of Schiff bases. Salicylic aldehydes are especially versatile in the latter case due to the enhanced stability of covalent adducts. Previously aldehydes were unpopular in drug discovery programs due to potential metabolic liability, but recent examples show surprisingly stable aldehydes passing clinical trials.
Cyanamides
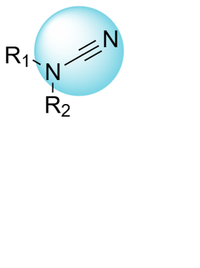
Cyanamides (or N-Nitriles) are a versatile class of electrophilic warheads capable of forming reversible covalent bonds with nucleophilic residues, particularly cysteines, through thiourea adduct formation. Unlike more reactive electrophiles, cyanamides offer a balance between stability and reactivity, reducing the risk of off-target effects.
Over 700 CyanamidesDisulfides
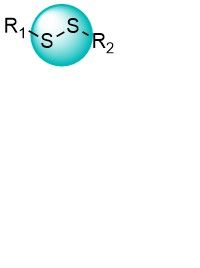
Disulfide moiety can form a covalent bond with Cys residues by the thiol-disulfide exchange. Disulfide bridges are an important feature of cysteines in biological systems. Disulfide tethering is a useful technique used for the identification of hit fragments.
Strained Cyclobutanes
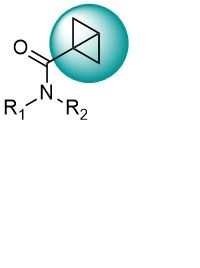
Bicyclo[1.1.0]butanes (BCB) recently emerged as a new class of thiol-reactive electrophiles and analogs of widely used acrylamides. Ring strain is utilized as a driving force for highly selective covalent tethering to nucleophilic amino acid residues. Hundreds of bicyclobutane carboxylic amides have been synthesized at Enamine and even more are to come from new syntheses
Over 500 Strained Cyclobutanes


