Publications
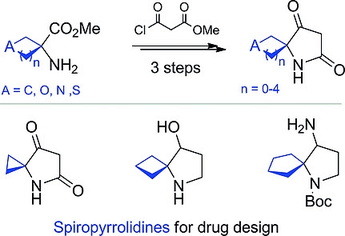
Eur. J. Org. Chem. 2019, (22), 3553-3559
DOI: 10.1002/ejoc.201801750
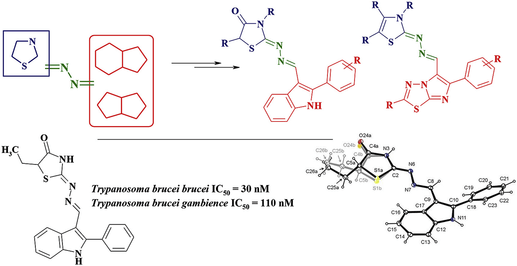
Eur. J. Med. Chem. 2019, 174, 292-308
DOI: 10.1016/j.ejmech.2019.04.052
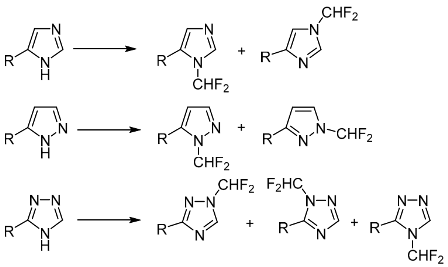
Chem. Heterocycl. Compd. 2019, 55 (4-5), 359-366
DOI: 10.1007/s10593-019-02465-x
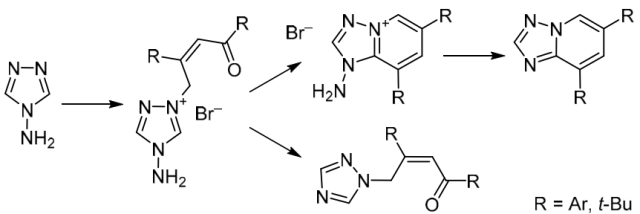
Chem. Heterocycl. Compd. 2019, 55 (4-5), 367-373
DOI: 10.1007/s10593-019-02466-w
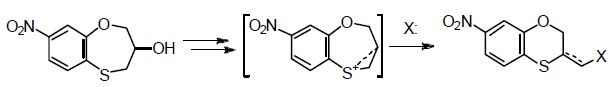
Chem. Heterocycl. Compd. 2019, 55 (4-5), 416-420
DOI: 10.1007/s10593-019-02474-w
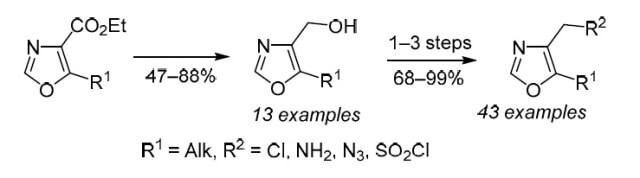
Chem. Heterocycl. Compd. 2019, 55 (4-5), 421-434
DOI: 10.1007/s10593-019-02475-9
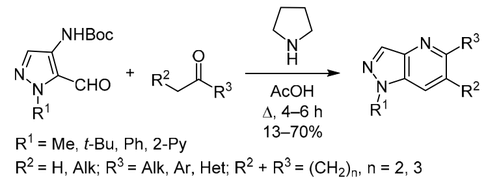
Chem. Heterocycl. Compd. 2019, 55 (4-5), 379-385
DOI: 10.1007/s10593-019-02468-8

An approach to the synthesis of 3-substituted piperidines bearing partially fluorinated alkyl groups
J. Fluorine Chem. 2019, 224, 61-66
DOI: 10.1016/j.jfluchem.2019.05.006

Eur. J. Org. Chem. 2019, (23), 3744-3750
DOI: 10.1002/ejoc.201900485
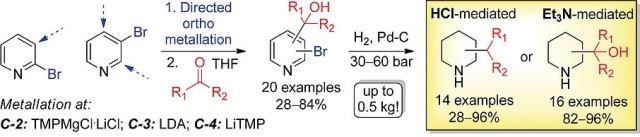
Eur. J. Org. Chem. 2019 (22), 3636-3648
DOI: 10.1002/ejoc.201900450
Beilstein J. Org. Chem. 2019, 15, 1032-1045
DOI: 10.3762/bjoc.15.101
ChemistrySelect 2019, 4 (17), 4933-4937
DOI: 10.1002/slct.201900650
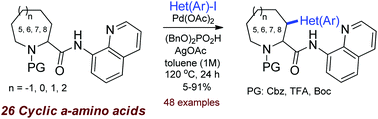
Org. Biomol. Chem. 2019, 17, 4342-4349
DOI: 10.1039/c9ob00393b

ACS Omega 2019, 4 (4), 7498-7515
DOI: 10.1021/acsomega.9b00896

Chem. Heterocycl. Compd. 2019, 55 (3), 196‑198
DOI: 10.1007/s10593-019-02440-6

J. Org. Chem. 2019, 84 (13), 8487-8496
DOI: 10.1021/acs.joc.9b00719

J. Am. Chem. Soc. 2019, 141 (16), 6726-6739
DOI: 10.1021/jacs.9b02238
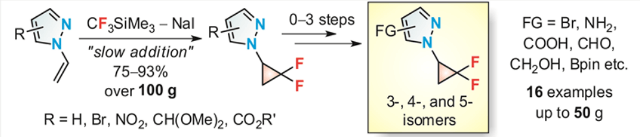
Eur. J. Org. Chem. 2019, (27), 4311-4319
DOI: 10.1002/ejoc.201900123
Molecules 2019, 24 (7), 1249-1260
DOI: 10.3390/molecules24071249

J. Heterocycl. Chem. 2019, 56 (5), 1605-1612
DOI: 10.1002/jhet.3541
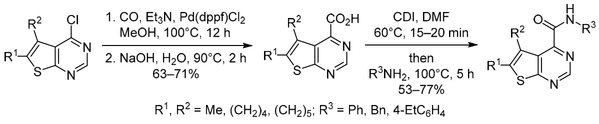
Chem. Heterocycl. Compd. 2019, 55 (2), 184‑188
DOI: 10.1007/s10593-019-02437-1
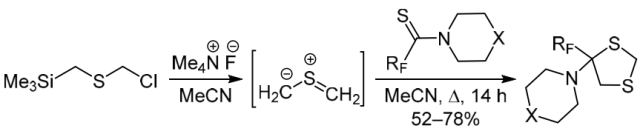
Chem. Heterocycl. Compd. 2019, 55 (2), 189‑192
DOI: 10.1007/s10593-019-02438-0

J. Fluorine Chem. 2019, 217, 80-89
DOI: 10.1016/j.jfluchem.2018.11.006

Chem. Heterocycl. Compd. 2019, 55 (3), 202‑204
DOI: 10.1007/s10593-019-02442-4
Regen. Med. 2019, 14 (3), 243-255
DOI: 10.2217/rme-2019-0001

Org. Lett. 2019, 21 (7), 2340-2345
DOI: 10.1021/acs.orglett.9b00622
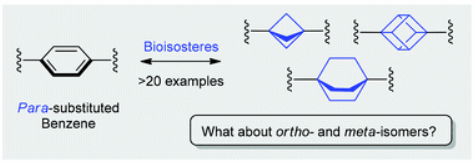
Org. Biomol. Chem. 2019, 17 (11), 2839-2849
DOI: 10.1039/c8ob02812e

Eur. J. Med. Chem. 2019, 165, 258-272
DOI: 10.1016/j.ejmech.2019.01.010
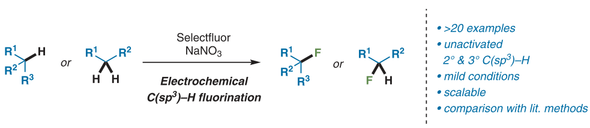
Synlett 2019, 30 (10), 1178-1182
DOI: 10.1055/s-0037-1611737
ACS Omega 2019, 4 (2), 2669−2675
DOI: 10.1021/acsomega.8b02388
Chemistry 2019, 25 (28), 6928-6940
DOI: 10.1002/chem.201900440
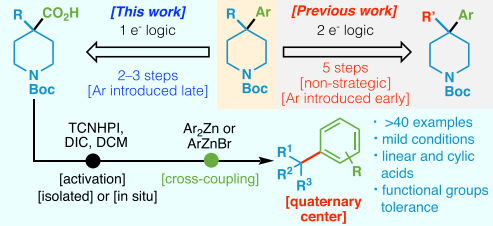
Angew. Chem. Int. Ed. 2019, 58 (8), 2454-2458
DOI: 10.1002/anie.201814524
Nature 2019, 566 (7743), 224-229
DOI: 10.1038/s41586-019-0917-9
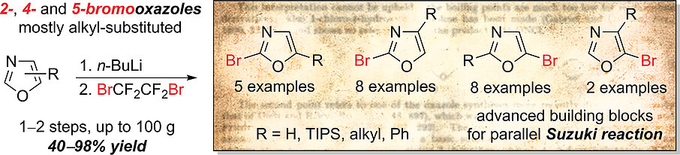
Eur. J. Org. Chem. 2019, (18), 2884-2898
DOI: 10.1002/ejoc.201900032
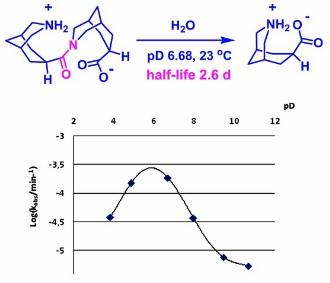
Molecules 2019, 24 (3), 572
DOI: 10.3390/molecules24030572
Drug Discovery Today 2019, 24 (2), 390-402
DOI: 10.1016/j.drudis.2018.10.016
New J. Chem. 2019, 43 (33), 13112-13121
DOI: 10.1039/c9nj01390c
Chem. Eur. J. 2019, 25 (24), 6053-6063
DOI: 10.1002/chem.201804953
Amino Acids 2019, 51 (2), 255-261
DOI: 10.1007/s00726-018-2660-1

J. Org. Chem. 2019, 84 (3), 1363-1371
DOI: 10.1021/acs.joc.8b02822
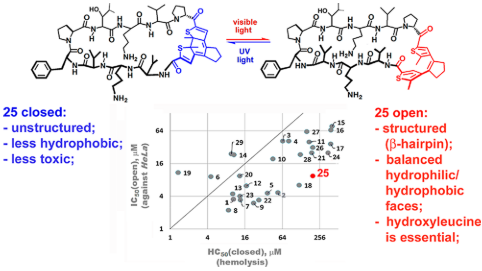
J. Med. Chem. 2018, 61 (23), 10793-10813
DOI: 10.1021/acs.jmedchem.8b01428
MolBank 2018, 4, 1022
DOI: 10.3390/M1022
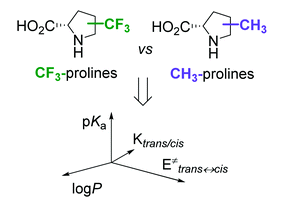
New J. Chem. 2018, 42 (16), 13461-13470
DOI: 10.1039/c8nj02631a

New J. Chem. 2018, 42 (21), 17499-17512
DOI: 10.1039/C8NJ03460E
Biopolym. Cell. 2018, 34 (1), 59-71
DOI: 10.7124/bc.000971

J. Org. Chem. 2018, 83 (12), 6275-6289
DOI: 10.1021/acs.joc.8b00077

Org. Biomol. Chem. 2018, 16 (44), 8559-8564
DOI: 10.1039/C8OB02428F
Heterocycl. Commun. 2018, 24 (4), 177-181
DOI: 10.1515/hc-2017-0143
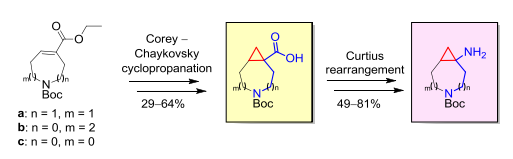
Tetrahedron Lett. 2018, 59 (52), 4611-4615
DOI: 10.1016/j.tetlet.2018.11.047
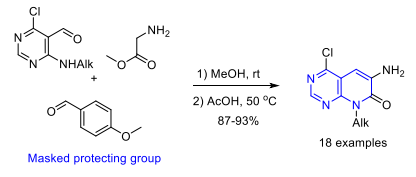
Eur. J. Org. Chem. 2018, 46, 6519-6523
DOI: 10.1002/ejoc.201801204
