Publications
J. Heterocycl. Chem. 2021, 58 (6), 1278-1285
DOI: 10.1002/jhet.4256

J. Org. Chem. 2021, 86 (11), 7333-7346
DOI: 10.1021/acs.joc.1c00148
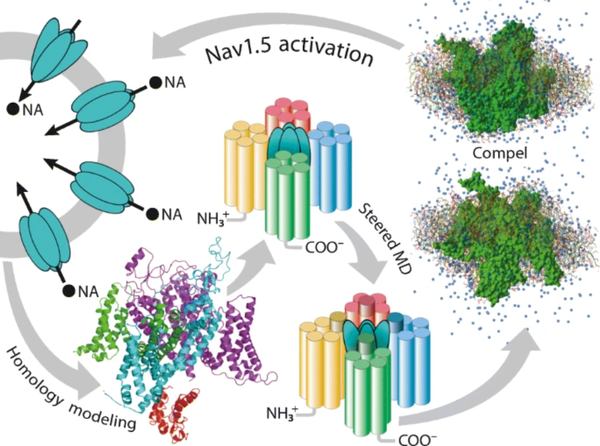
J. Mol. Model. 2021, 27 (6), 182
DOI: 10.1007/s00894-021-04799-w
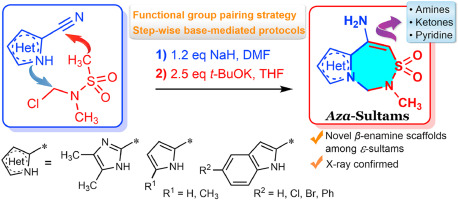
Tetrahedron 2021, 88, 132149
DOI: 10.1016/j.tet.2021.132149
Sci. Pharm. 2021, 89(2), 22
DOI: 10.3390/scipharm89020022

ACS Omega 2021, 6 (15), 10119–10128
DOI: 10.1021/acsomega.1c00193
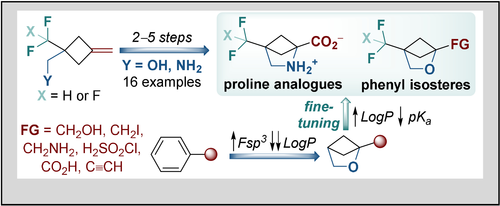
Eur. J. Org. Chem. 2021, 2021 (47), 6580-6590
DOI: 10.1002/ejoc.202100414
Arch. Pharm. 2021, 354 (4), e2000275
DOI: 10.1002/ardp.202000275

Chem. Heterocycl. Compd. 2021, 57 (2), 199-206
DOI: 10.1007/s10593-021-02893-8

iScience 2021, 24 (2), 102021
DOI: 10.1016/j.isci.2020.102021

Eur. J. Org. Chem. 2021, 2021 (17), 2313-2330
DOI: 10.1002/ejoc.202100195
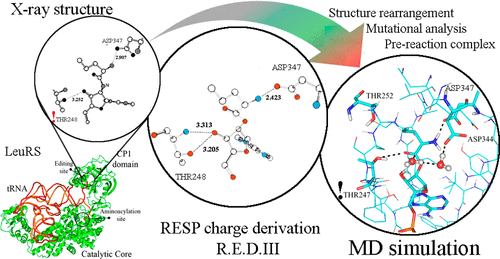
ACS Omega 2021, 6 (6), 4227-4235
DOI: 10.1021/acsomega.0c05143

Molecules 2021, 26 (4), 1021
DOI: 10.3390/molecules26041021

Chem. Rev. 2021, 121 (3), 1670–1715
DOI: 10.1021/acs.chemrev.0c01015
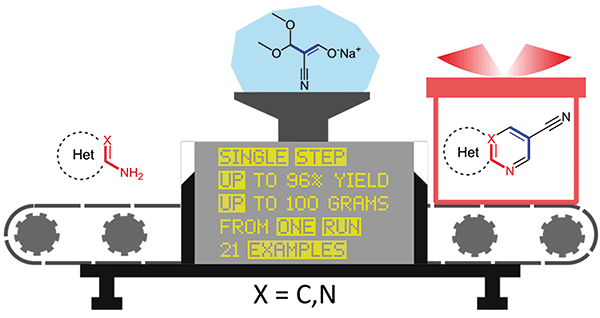
Synthesis 2021, 53 (12), 2133-2141
DOI: 10.1055/a-1360-9852
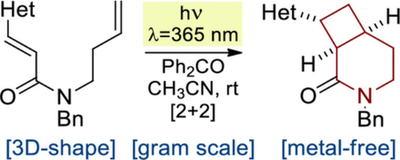
J. Org. Chem. 2021, 86 (3), 2200-2209
DOI: 10.1021/acs.joc.0c02355

J. Chem. Inf. Model. 2021, 61 (1), 179-188
DOI: 10.1021/acs.jcim.0c00936
Chem. Nat. Compd. 2021, 57 (1), 33-37
DOI: 10.1007/s10600-021-03275-4
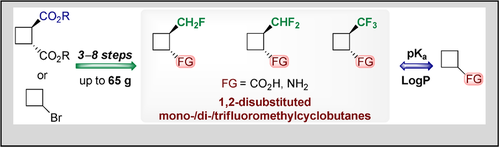
Eur. J. Org. Chem. 2021, 2021 (1), 87-95
DOI: 10.1002/ejoc.202001345
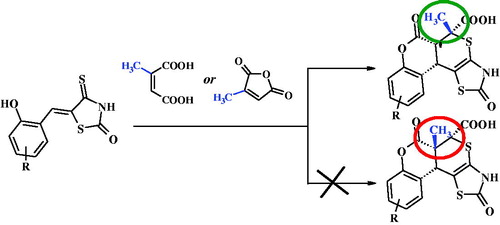
Synth. Commun. 2021, 51 (6), 964-970
DOI: 10.1080/00397911.2020.1867868
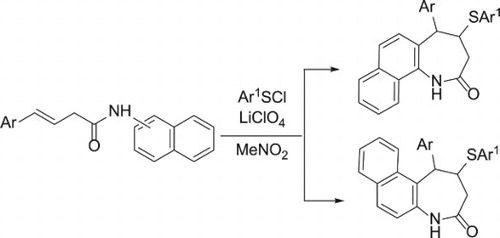
J. Sulphur Chem. 2021, 42 (3), 264-280
DOI: 10.1080/17415993.2020.1855431
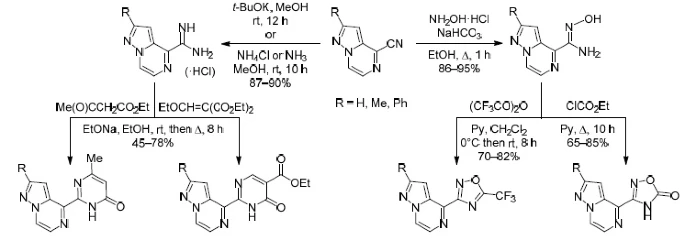
Chem. Heterocycl. Compd. 2020, 56 (12), 1554-1559
DOI: 10.1007/s10593-020-02849-4
J. Extracell. Vesicles 2020, 10 (2), e12039
DOI: 10.1002/jev2.12039
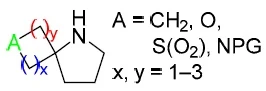
Chem. Heterocycl. Compd. 2020, 56 (11), 1411-1413
DOI: 10.1007/s10593-020-02829-8
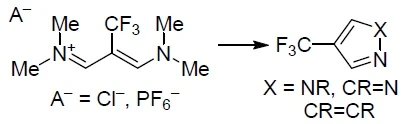
Chem. Heterocycl. Compd. 2020, 56 (11), 1408-1410
DOI: 10.1007/s10593-020-02828-9
Theor. Exp. Chem. 2020, 56 (5), 283-308
DOI: 10.1007/s11237-020-09660-4
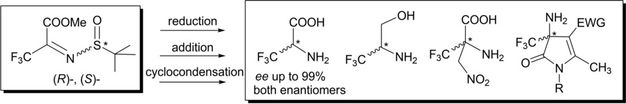
ChemistrySelect 2020, 5 (43), 13569-13574
DOI: 10.1002/slct.202003500

Chem. Heterocycl. Compd. 2020, 56 (10), 1329-1334
DOI: 10.1007/s10593-020-02818-x
Scientific Data 2020, 7 (1), 384
DOI: 10.1038/s41597-020-00727-4
Cells 2020, 9 (10), 2272
DOI: 10.3390/cells9102272
Theor. Exp. Chem. 2020, 56 (4), 261-267
DOI: 110.1007/s11237-020-09657-z
Angew. Chem. Int. Ed. 2020, 59 (41), 18016‑18022
DOI: 10.1002/anie.202007651
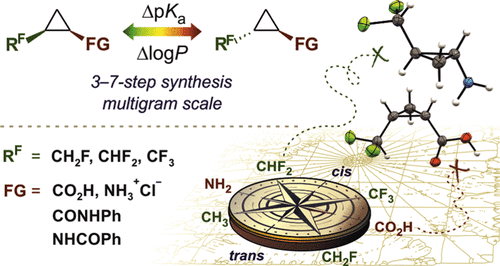
J. Org. Chem. 2020, 85 (19), 12692-12702
DOI: 10.1021/acs.joc.0c01848

Chem. Heterocycl. Compd. 2020, 56 (8), 1048‑1053
DOI: 10.1007/s10593-020-02771-9
Beilstein J. Org. Chem. 2020, 16, 2304-2313
DOI: 10.3762/bjoc.16.191
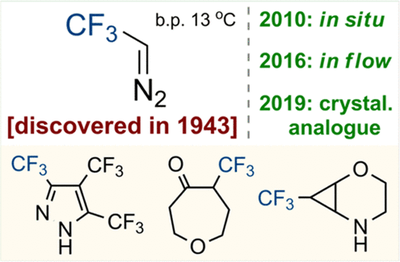
Chem. Rev. 2020, 120 (22), 12718-12755
DOI: 10.1021/acs.chemrev.0c00406

Org. Process Res. Dev. 2020, 24 (11), 2619‑2632
DOI: 10.1021/acs.oprd.0c00300
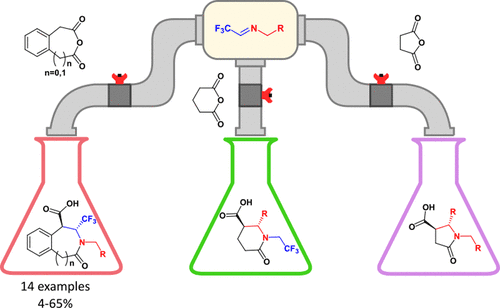
ACS Omega 2020, 5 (33), 20932-20942
DOI: 10.1021/acsomega.0c02394
Org. Biomol. Chem. 2020, 18 (28), 5359-5369
DOI: 10.1039/d0ob00831a
Chem. Heterocycl. Compd. 2020, 56 (6), 719-721
DOI: 10.1007/s10593-020-02721-5
Angew. Chem. Int. Ed. 2020, 59 (46), 20515‑20521
DOI: 10.1002/anie.202004183
J. Cell. Biochem. 2020, 121 (12), 4922-4930
DOI: 10.1002/jcb.29820
J. Heterocycl. Chem. 2020, 57(8), 3202‑3212
DOI: 10.1002/jhet.4028
Z. Anorg. Allg. Chem. 2020, 646 (20), 1710‑1714
DOI: 10.1002/zaac.201900319
J. Biomol. NMR 2020, 74 (10-11), 555–563
DOI: 10.1007/s10858-020-00327-9

Advanced Synthesis and Catalysis 2020, 362 (15), 3229-3242
DOI: 10.1002/adsc.202000450
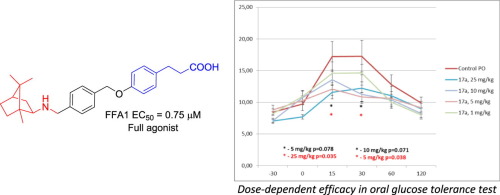
Bioorg. Chem. 2020, 99, 103830
DOI: 10.1016/j.bioorg.2020.103830

ChemistrySelect 2020, 5 (19), 5573-5576
DOI: 10.1002/slct.202001243
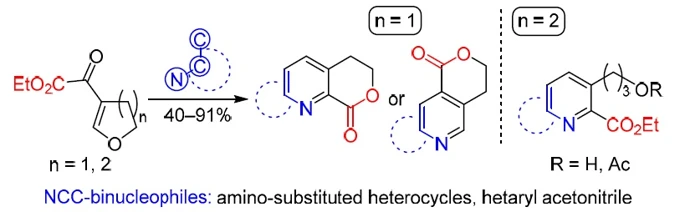
Chem. Heterocycl. Compd. 2020, 56 (3), 377–385
DOI: 10.1007/s10593-020-02670-z
Eur. J. Org. Chem. 2020, 2020 (22), 3261-3269
DOI: 10.1002/ejoc.202000314
