Bioorg. Chem. 2020, 99, 103830
DOI: 10.1016/j.bioorg.2020.103830
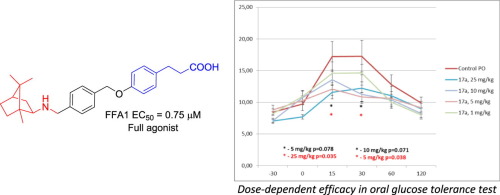
Six derivatives of 3-phenylpropionic acid bearing various natural and natural-like, spatially defined peripheral motifs have been synthesized and evaluated in vitro for free fatty acid receptor 1 (FFA1) activation. Two frontrunner compounds (bearing a bornyl and cytosine groups) were evaluated in an oral glucose tolerance test in mice where both demonstrated the ability to sustain blood glucose levels following a glucose challenge. The bornyl compound displayed a somewhat superior, dose-dependent efficacy and, therefore, can be regarded as a lead compounds for further development as a therapeutic agent for type 2 diabetes mellitus. Its high affinity to FFA1 was rationalized by docking experiments.