Publications
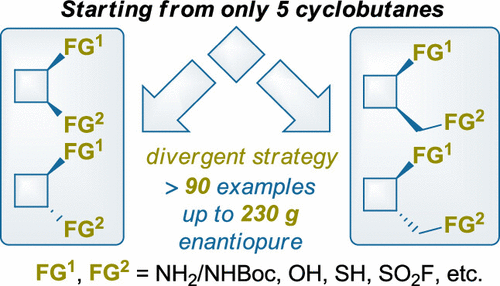
J. Org. Chem. 2023, 88 (5), 3109-3131
DOI: 10.1021/acs.joc.2c02892
ChemRxiv 2023, in press
DOI: 10.26434/chemrxiv-2023-jbb0r

Molecules 2023, 28 (3), 1201
DOI: 10.3390/molecules28031201
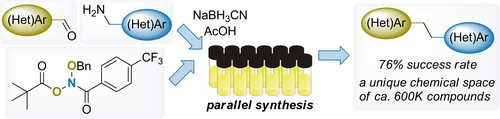
Chem. Eur. J. 2023, 29 (4), e202203470
DOI: 10.1002/chem.202203470
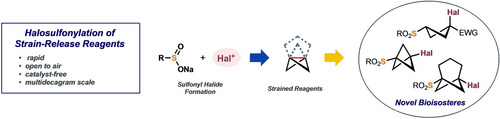
Angew. Chem. Int. Ed. 2023, 62 (3), e202213508
DOI: 10.1002/anie.202213508
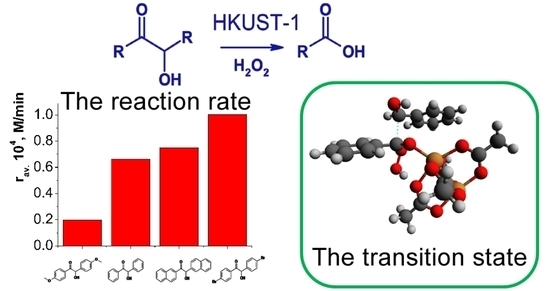
Molecules 2023, 28 (2), 747
DOI: 10.3390/molecules28020747
Small. 2022, 18 (41), e2107308
DOI: 10.1002/smll.202107308
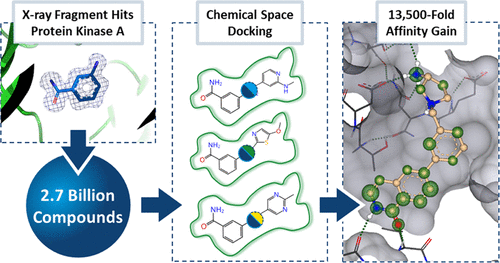
J. Med. Chem. 2022, 65 (23), 15663-15678
DOI: 10.1021/acs.jmedchem.2c00813
Proc. Natl. Acad. Sci. U.S.A. 2023, 120 (2), e2212931120
DOI: 10.1073/pnas.2212931120
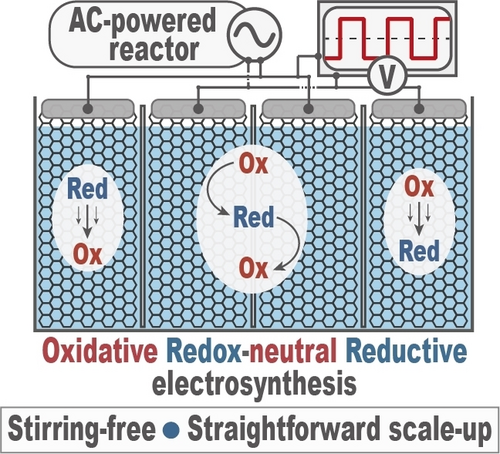
Chemistry 2023, 29 (18), e202203825
DOI: 10.1002/chem.202203825
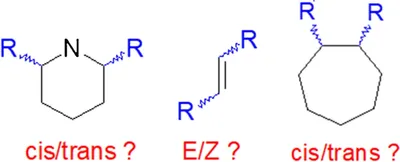
Anal. Bioanal. Chem. 2023, 415 (1), 3-4
DOI: 10.1007/s00216-022-04383-y
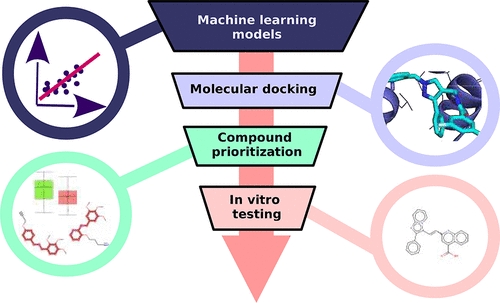
J. Chem. Inf. Model. 2022, 62, 24, 6323–6335
DOI: 10.1021/acs.jcim.1c01460
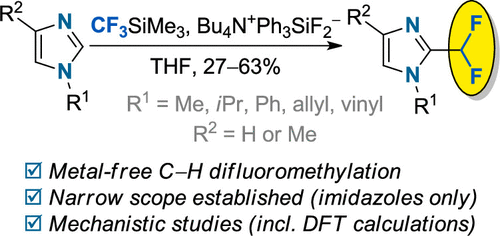
J. Org. Chem. 2023, 88 (1), 163-171
DOI: 10.1021/acs.joc.2c02041
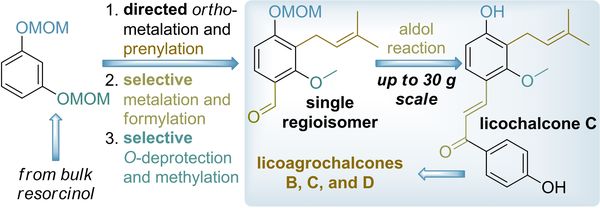
Eur. J. Org. Chem. 2022, 26 (3), e202201226
DOI: 10.1002/ejoc.202201226

Org. Biomol. Chem. 2022, 20 (47), 9337-9350
DOI: 10.1039/d2ob01430k
Challenges for chemistry in Ukraine after the war: Ukrainian science requires rebuilding and support
Proc. Natl. Acad. Sci. U.S.A. 2022, 119 (50), e2210686119
DOI: 10.1073/pnas.2210686119
Chem. Biol. Drug. Des. 2022, 100 (6), 1025-1032
DOI: 10.1111/cbdd.13975
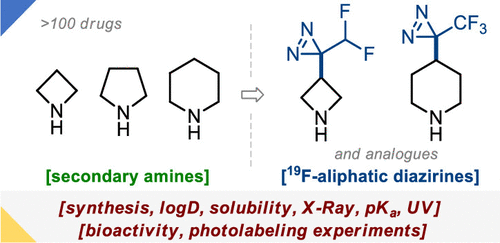
J. Org. Chem. 2023, 88 (1), 1-17
DOI: 10.1021/acs.joc.2c02262
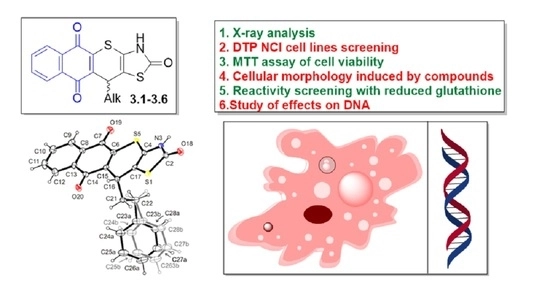
Molecules 2022, 27 (21), 7575
DOI: 10.3390/molecules27217575
ChemMedChem 2022, 17 (21), e202200365
DOI: 10.1002/cmdc.202200365
Acta Cryst. E 2022, 78 (12), 1156-1160
DOI: 10.1107/s2056989022010362
Molbank 2022, 2022 (4), 1478
DOI: 10.3390/m1478
J. Comput. Chem. 2023, 44 (2), 76-92
DOI: 10.1002/jcc.27016
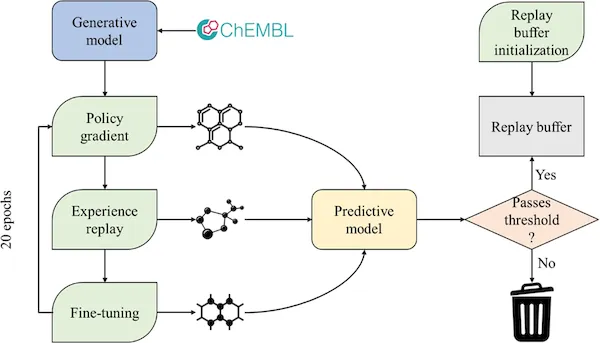
Commun. Chem. 2022, 5 (1), 129
DOI: 10.1038/s42004-022-00733-0
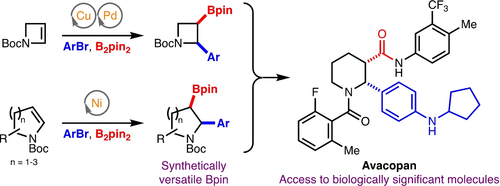
Angew. Chem. Int. Ed. 2022, 61 (46), e202212117
DOI: 10.1002/anie.202212117

J. Colloid Interface Sci. 2022, 624, 270-278
DOI: 10.1016/j.jcis.2022.05.124
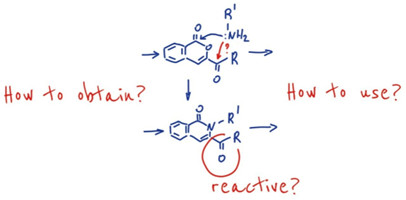
Arkivoc 2022, 2022 (8), 79-112
DOI: 10.24820/ark.5550190.p011.861
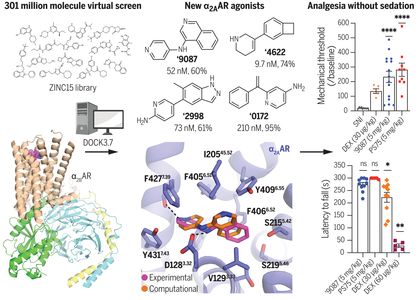
Science 2022, 377 (6614), eabn7065
DOI: 10.1126/science.abn7065
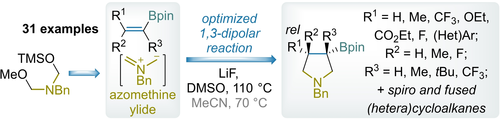
Chem. Eur. J. 2022, 28 (54), e202202117
DOI: 10.1002/chem.202202117
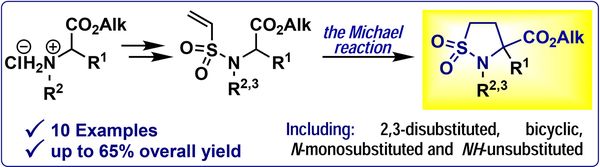
Tetrahedron 2022, 124 133013
DOI: 10.1016/j.tet.2022.133013
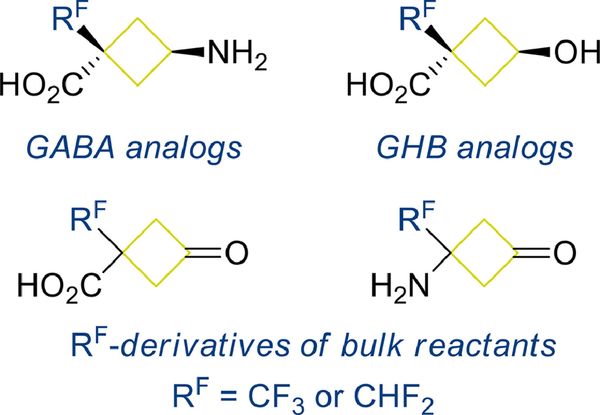
J. Fluorine Chem. 2022, 263 110041
DOI: 10.1016/j.jfluchem.2022.110041
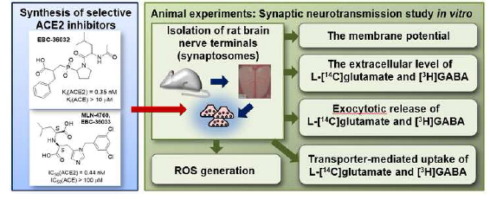
Neuroscience 2022, 498, 155-173
DOI: 10.1016/j.neuroscience.2022.07.003
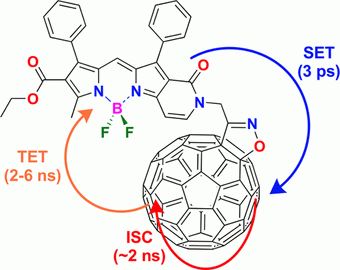
J. Phys. Chem. Lett. 2022, 13 (38), 8845-8850
DOI: 10.1021/acs.jpclett.2c02388

J. Am. Chem. Soc. 2022, 144 (38), 17709-17720
DOI: 10.1021/jacs.2c08006
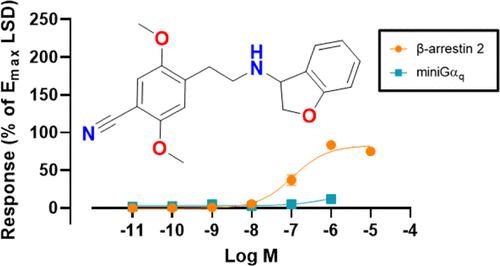
J. Med. Chem. 2022, 65 (18), 12031-12043
DOI: 10.1021/acs.jmedchem.2c00702
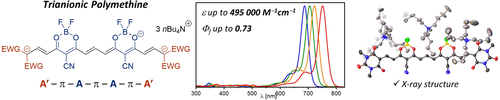
Chem. Eur. J. 2022, 28 (70), e202202168
DOI: 10.1002/chem.202202168
Molecules 2022, 27 (17), 5400
DOI: 10.3390/molecules27175400
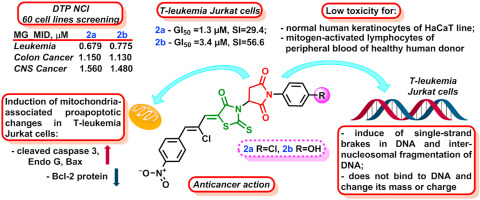
Eur. J. Med. Chem. 2022, 238, 114422
DOI: 10.1016/j.ejmech.2022.114422
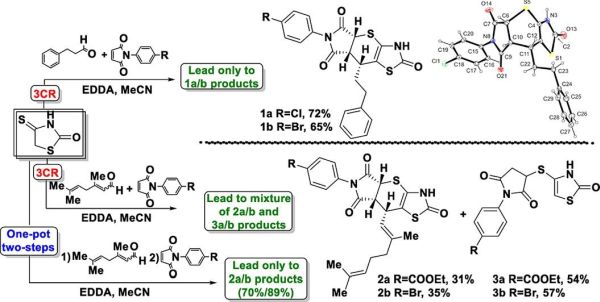
Results Chem. 2022, 4, 100464
DOI: 10.1016/j.rechem.2022.100464
Acta Crystallogr E Crystallogr Commun 2022, 78, 829-832
DOI: 10.1107/S2056989022007460
Rejuvenation Research 2022, 25 (4), 191-199
DOI: 10.1089/rej.2022.0029
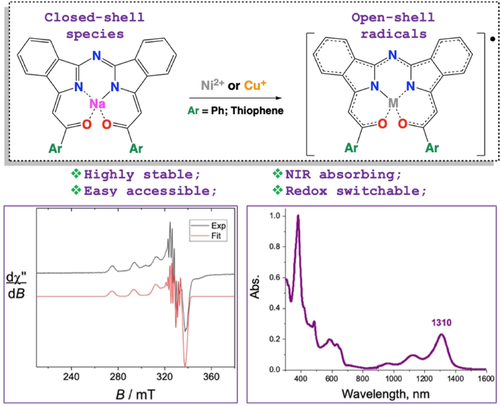
Chem. Eur. J. 2022, 28 (41), e202201181
DOI: 10.1002/chem.202201181
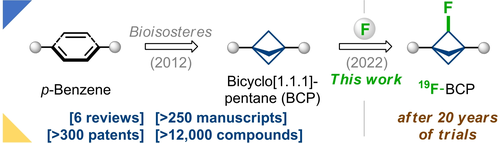
Angew. Chem. Int. Ed. 2022, 61 (29), e202205103
DOI: 10.1002/anie.202205103

ACS Med. Chem. Lett. 2022, 13 (7), 992-996
DOI: 10.1021/acsmedchemlett.2c00211

Chemistry 2022, 28 (54), e202201261
DOI: 10.1002/chem.202201261
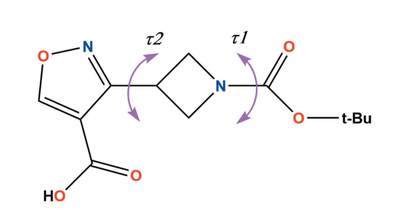
Acta Crystallogr. B: Struct. Sci. Cryst. Eng. Mater. 2022, 78, 510-519
DOI: 10.1107/S2052520622003900
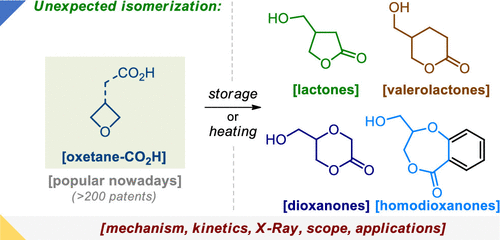
Org. Lett. 2022, 24 (26), 4722-4728
DOI: 10.1021/acs.orglett.2c01402

Chem. Eur. J. 2022, 28 (55), e202201601
DOI: 10.1002/chem.202201601
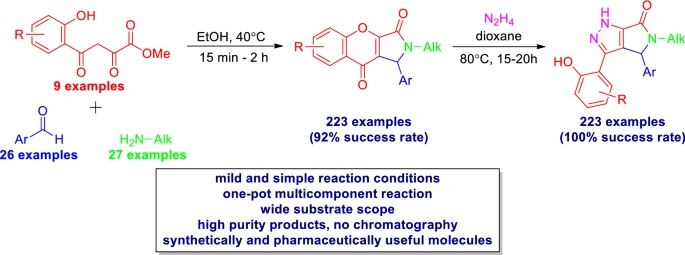
Mol. Divers. 2022, 26 (2), 1115-1128
DOI: 10.1007/s11030-021-10234-2
Science 2022, 376 (6596), 929
DOI: 10.1126/science.abq7841
