Publications
Chem. Eur. J. 2022, 28 (19), e202200331
DOI: 10.1002/chem.202200331
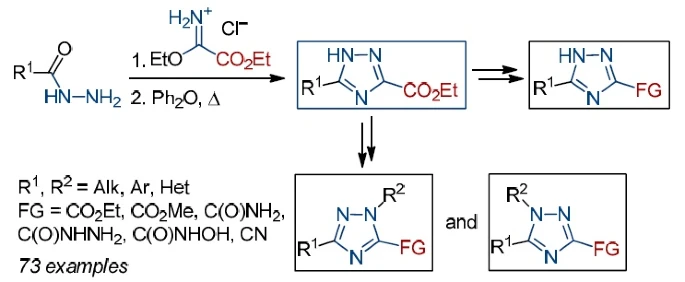
Chem. Heterocycl. Compd. 2022, 58 (2-3), 116-128
DOI: 10.1007/s10593-022-03064-z

Org. Biomol. Chem. 2022, 20 (15), 3183-3200
DOI: 10.1039/d2ob00146b

ChemMedChem 2022, 17 (7), e202100641
DOI: 10.1002/cmdc.202100641
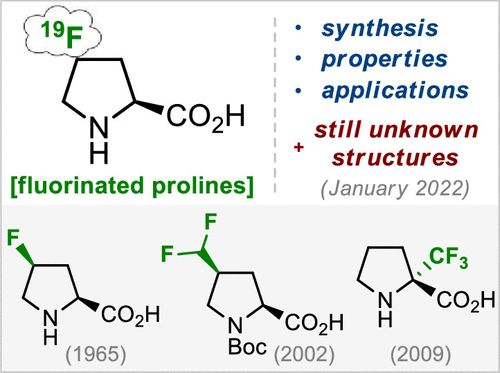
J. Org. Chem. 2022, 87 (11), 6961-7005
DOI: 10.1021/acs.joc.1c02956

Chem. Sci. 2022, 13, 3674-3687
DOI: 10.1039/d1sc05892d
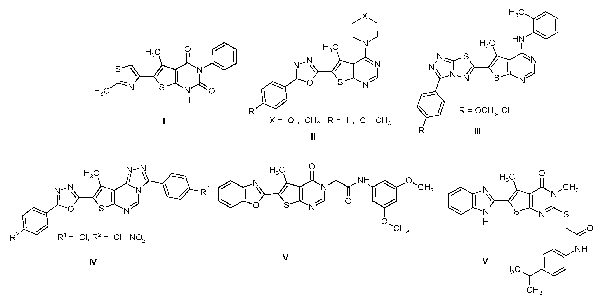
Molbank 2022, 2022 (1), M1331
DOI: 10.3390/m1331
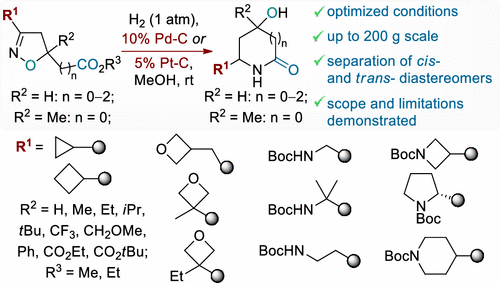
J. Org. Chem. 2022, 87 (2), 1001–1018
DOI: 10.1021/acs.joc.1c02301
Nature 2022, 601 (7893), 452-459
DOI: 10.1038/s41586-021-04220-9
Mol Inform 2022, 41 (6), e2100289
DOI: 10.1002/minf.202100289
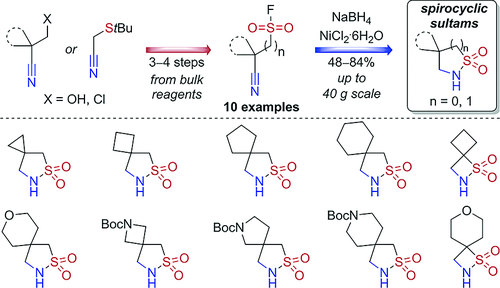
Eur. J. Org. Chem. 2021, 2021 (47), 6530‑6540
DOI: 10.1002/ejoc.202000351
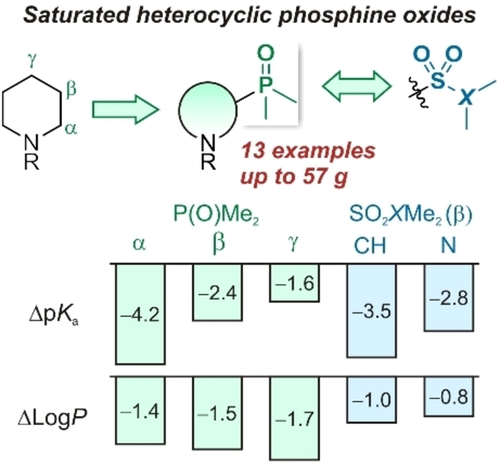
Eur. J. Org. Chem. 2021, 2021 (47), 6591‑6603
DOI: 10.1002/ejoc.202100581
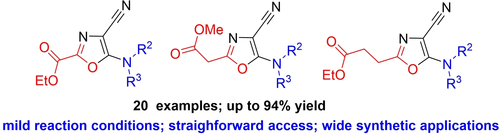
Eur. J. Org. Chem. 2021, 2021 (47), 6511‑6523
DOI: 10.1002/ejoc.202100412
Eur. J. Org. Chem. 2021, 2021 (47), 6478-6510
DOI: 10.1002/ejoc.202100857
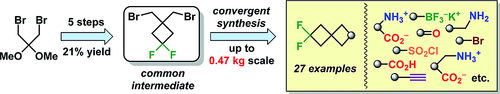
Eur. J. Org. Chem. 2021, 2021 (47), 6541-6550
DOI: 10.1002/ejoc.202000432
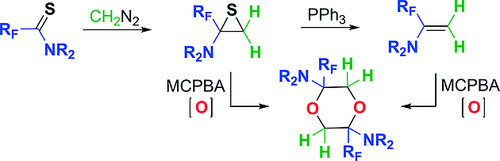
Eur. J. Org. Chem. 2021, 2021 (47), 6524-6529
DOI: 10.1002/ejoc.202001104
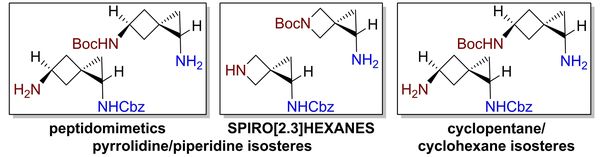
Eur. J. Org. Chem. 2021, 2021 (47), 6570-6579
DOI: 10.1002/ejoc.202001614
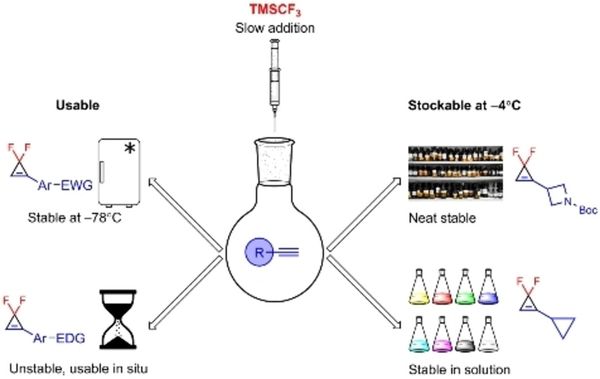
Eur. J. Org. Chem. 2021, 2021 (47), 6604-6615
DOI: 10.1002/ejoc.202100921
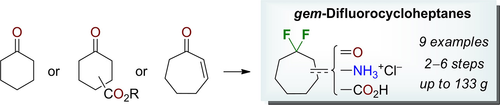
Eur. J. Org. Chem. 2021, 2021 (47), 6561-6569
DOI: 10.1002/ejoc.202001530

Eur. J. Org. Chem. 2021, 2021 (47), 6474-6477
DOI: 10.1002/ejoc.202101210
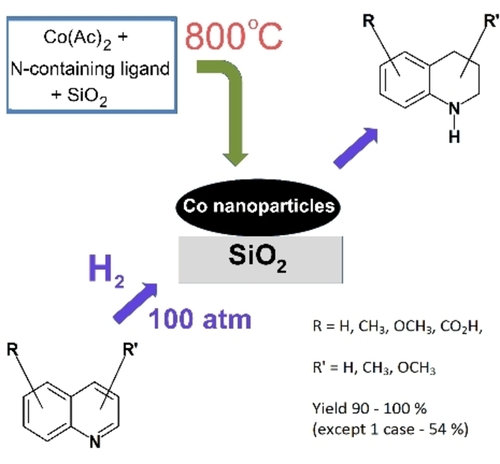
Eur. J. Org. Chem. 2021, 2021 (47), 6616-6625
DOI: 10.1002/ejoc.202101311
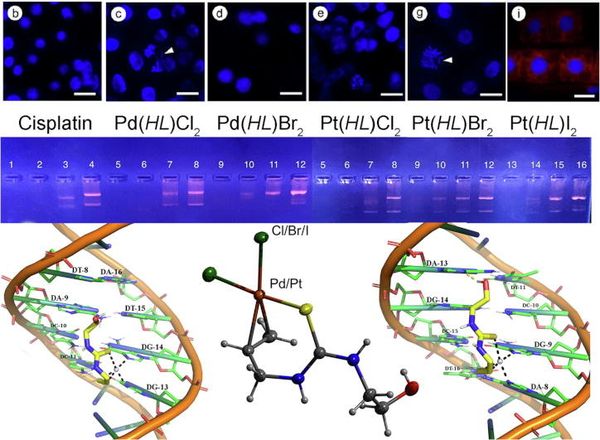
Polyhedron 2021, 210 115477
DOI: 10.1016/j.poly.2021.115477
Beilstein J. Org. Chem. 2021, 17 2787-2794
DOI: 10.3762/bjoc.17.189
Sci. Pharm. 2021, 89(4), 49
DOI: 10.3390/scipharm89040049
Acta Cryst. 2021, 77, 1285-1288
DOI: 10.1107/S2056989021011865
Acta Cryst. 2021, 77, 1153-1157
DOI: 10.1107/S2056989021010653
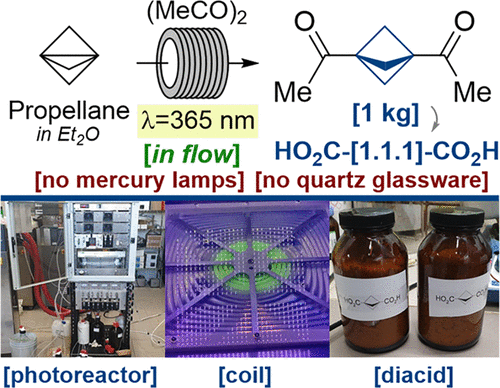
J. Org. Chem. 2021, 86 (20), 14061–14068
DOI: 10.1021/acs.joc.1c00977
J. Heterocycl. Chem. 2021, 59 (2), 329-340
DOI: 10.1002/jhet.4387
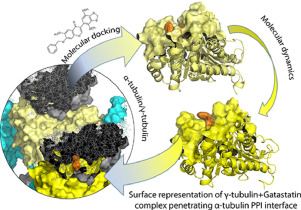
J. Mol. Struct. 2021, 1241, 130633
DOI: 10.1016/j.molstruc.2021.130633
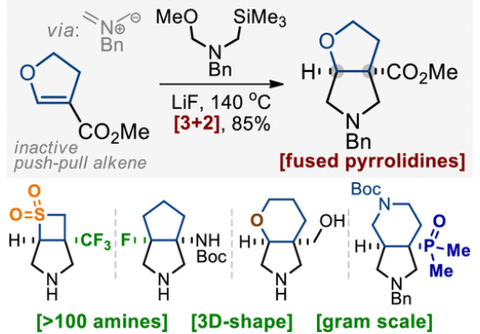
J. Org. Chem. 2021, 86 (19), 13289–13309
DOI: 10.1002/slct.202001243
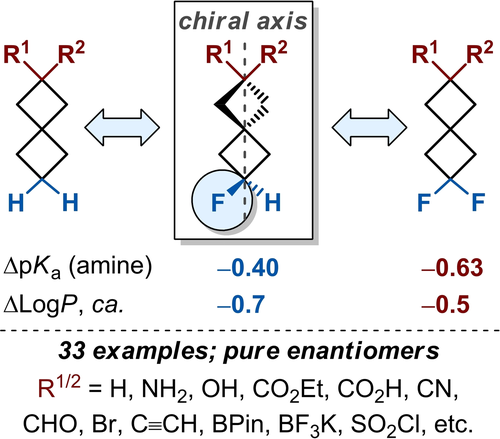
Eur. J. Org. Chem. 2021, 2021 (35), 4897-4910
DOI: 10.1002/ejoc.202100804

Chem. Heterocycl. Compd. 2021, 57 (7-8), 841-847
DOI: 10.1007/s10593-021-02989-1
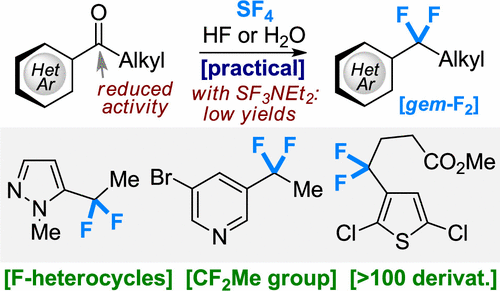
J. Org. Chem. 2021, 86 (17), 12181–12198
DOI: 10.1021/acs.joc.1c01518
Chem. Sci. 2021, 12 (34), 11294-11305
DOI: 10.1039/d1sc03615g
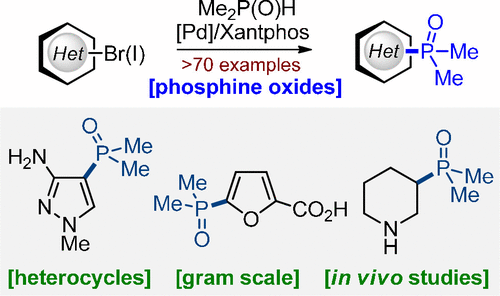
J. Org. Chem. 2021, 86, 18, 12783–12801
DOI: 10.1021/acs.joc.1c01413
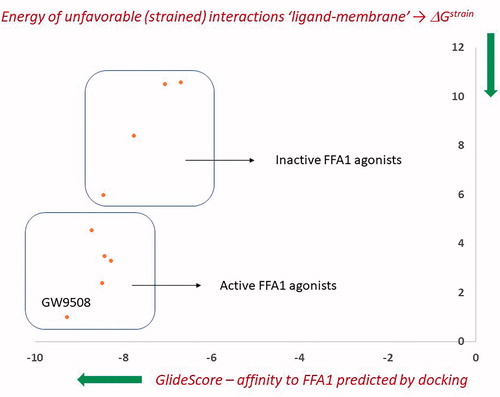
J. Enzyme Inhib. Med. Chem. 2021, 36 (1), 1651-1658
DOI: 10.1080/14756366.2021.1955874
Life 2021, 11(7), 659
DOI: 10.3390/life11070659
Front. Chem. 2021, 9, 688446
DOI: 10.3389/fchem.2021.688446
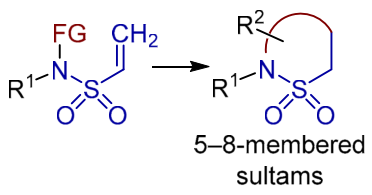
Chem. Heterocycl. Compd. 2021, 57 (6), 630-632
DOI: 10.1007/s10593-021-02960-0
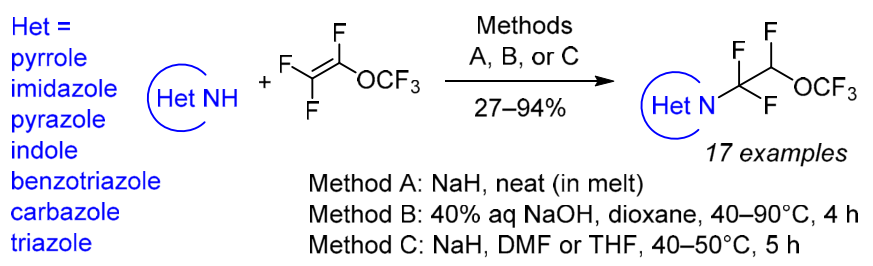
Chem. Heterocycl. Compd. 2021, 57 (6), 666-671
DOI: 10.1007/s10593-021-02965-9

Chem Sci 2021, 12(28), 9694‑9703
DOI: 10.1039/d1sc01916c
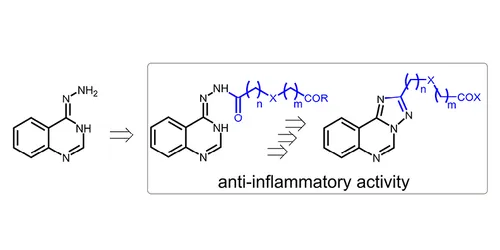
Acta Chim. Slov. 2021, 68 (2), 395-403
DOI: 10.17344/acsi.2020.6440
Molecules 2021, 26(12), 3507
DOI: 10.3390/molecules26123507
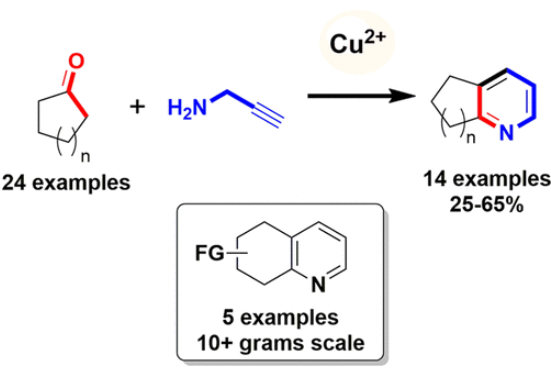
J. Org. Chem. 2021, 86 (11), 7315-7325
DOI: 10.1021/acs.joc.0c03038
J. Heterocycl. Chem. 2021, 58 (6), 1278-1285
DOI: 10.1002/jhet.4256

J. Org. Chem. 2021, 86 (11), 7333-7346
DOI: 10.1021/acs.joc.1c00148
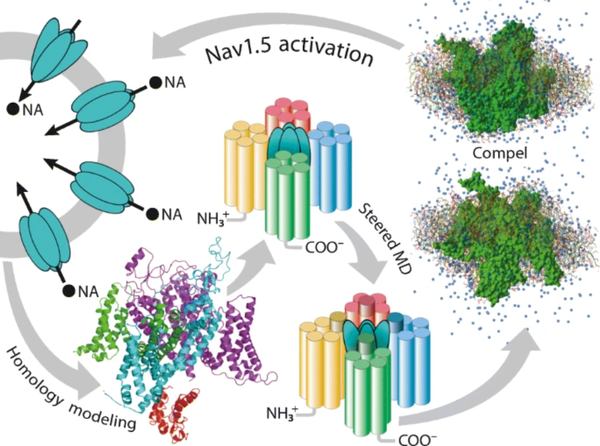
J. Mol. Model. 2021, 27 (6), 182
DOI: 10.1007/s00894-021-04799-w
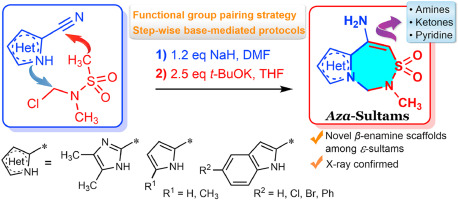
Tetrahedron 2021, 88, 132149
DOI: 10.1016/j.tet.2021.132149
Sci. Pharm. 2021, 89(2), 22
DOI: 10.3390/scipharm89020022

ACS Omega 2021, 6 (15), 10119–10128
DOI: 10.1021/acsomega.1c00193
