J. Mol. Struct. 2021, 1241, 130633
DOI: 10.1016/j.molstruc.2021.130633
Microtubules and actin filaments are cytoskeletal protein assemblies that are engaged in motility, mitosis, and regulate cell signalling with an essential role in the control of protein synthesis and accordingly, cell growth. Inhibition of cytoskeletal organelles, in particular tubulin polymerization, is important in cancer therapy due to their antimitotic role.
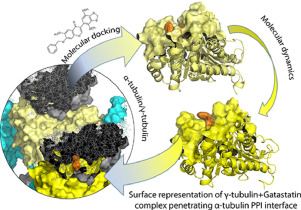
Considering protein structure variations, we conducted a molecular docking study followed by a series of molecular dynamics (MD) simulations at different binding sites to investigate how a known α-/β-tubulin dimerization inhibitor, Glaziovianin A, and its analogue Gatastatin target human γ-tubulin subunit. The results of our MD simulations reproduced these protein-ligand interactions, demonstrating that Glaziovianin A is selective against β-tubulin's colchicine site and Gatastatin hits γ-tubulin's GTP binding site.
Further analyses shed light on the reasons for subunit-focused selectivity for these structurally similar compounds, provided mechanistic insights into some of the tubulin functions such as the conformational transitions and subsequent microtubule disruption. The information combined from energy and structural analysis of MD, with all represented validation steps, and detailed study of specific protein-protein interaction features potentially could facilitate the early identification of γ-tubulin-specific inhibitors.