Eur. J. Org. Chem. 2018, 22, 2753-2761
DOI: 10.1002/ejoc.201800311
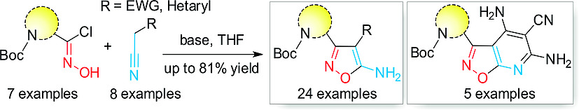
The reaction of chloroximes that have a protected amino group and active methylene nitriles under basic conditions has been shown to give functionalized 5‐aminoisoxazoles in yields of 37–81 %. This method has a broad substrate scope, including nitriles that contain either an electron‐withdrawing (i.e., CO2Me, CN, or SO2Me) or a heteroaryl group at the α‐position. Moreover, a malononitrile dimer was employed in analogous transformations, which led to the formation of polyfunctional isoxazolo[5,4‐b]pyridines. The selective removal of the protecting groups contained in the product demonstrated the utility of these compounds as advanced building blocks in early drug discovery programs.