ACS Comb. Sci. 2018, 20 (1), 35-43
DOI: 10.1021/acscombsci.7b00163
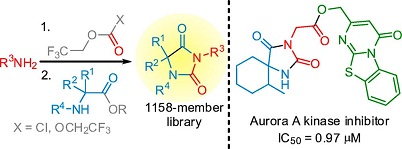
An approach to the parallel synthesis of hydantoin libraries by reaction of in situ generated 2,2,2-trifluoroethylcarbamates and μ-amino esters was developed. To demonstrate utility of the method, a library of 1158 hydantoins designed according to the lead-likeness criteria (MW 200-350, cLogP 1-3) was prepared. The success rate of the method was analyzed as a function of physicochemical parameters of the products, and it was found that the method can be considered as a tool for lead-oriented synthesis. A hydantoin-bearing submicromolar primary hit acting as an Aurora kinase A inhibitor was discovered with a combination of rational design, parallel synthesis using the procedures developed, in silico and in vitro screenings.