Heterocycl. Commun. 2017, 23 (6), 449–453
DOI: 10.1515/hc-2017-0180
Subota A.; Volochnyuk D.; Gorlova A.; Grygorenko O.
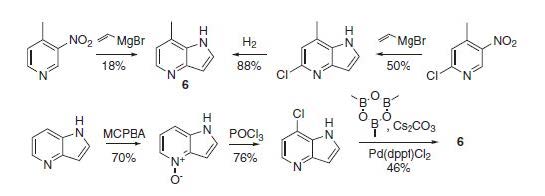
An approach to the synthesis of 7-methyl-4-azaindole, which is a valuable building block for drug discovery programs, is described. The method relies on using a bromine atom as a ‘place holding group’ for one of the carbon atoms of the pyridine ring throughout the reaction sequence, and it is removed only upon the final reductive cyclization leading to the azaindole ring. Exhaustive hydrogenation of the target product proceeds in a diastereoselective manner and leads to a bicyclic conformationally restricted diamine derivative.