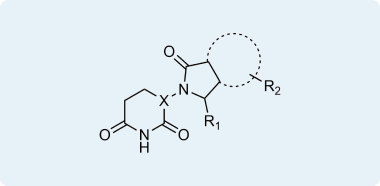
CRBN binders and their functionalized analogs
Cereblon (CRBN) is one of the most explored E3 ligases extensively used to construct PROTACs, search for new Molecular Glues, and develop functionalized tool compounds. The most extensive research has been done in this area. CRBN ligands are also known to have a good ADME profile, allowing for the development of orally bioavailable PROTACs.
We have been working with Thalidomide-like and Glutarimide chemistry for years and gained versatile experience in this chemistry field. Our experienced chemists are continuously working on the design and synthesis of new attractive Building Blocks, while our parallel chemistry group extensively elaborates approaches in the modification of the most interesting intermediates. This knowledge allowed us to enumerate a REAL Array of over 18 million CRBN-focused compounds, which can be synthesized within only 4 weeks and with 80% success rate.
Screening Compounds and Libraries
Over 15 000 CRBN focused compounds are available from stock including Glutarimides, IMiD Library Uracil-like, and Avadomide derivatives. All products are constantly updated with newly synthesized compounds. Covalent modifiers are represented by diverse and the most reliable warheads focusing on Cys, Lys, and Ser residues.
Download SD files
Plated
Stock
REAL
Product catalog
IMiD Library
IMiD-4900
Size
4 900
compounds
Descriptions
Designed and specially synthesized for the discovery of Immunomodulatory Imide Drugs
Download file
CRBN-MG-4560
Size
4 560
compounds
Description
Designed to capture the widest possible range of novel chemotypes of CRBN binders, providing a foundation for the discovery of next-generation Molecular Glues
Download file
PAG-640
Size
640
compounds
Description
Built upon novel Phenyl Amino Glutarimide (PAG) analogs, these compounds combine structural innovation with potent activity — offering a fresh perspective in Molecular Glue design
Download file
PD-880
Size
880
compounds
Description
Chemical stability, cellular potency, and permeability are just a few of the advantages that make Phenyl Dihydrouracil (PD)-based CRBN ligands promising candidates for optimal Molecular Glue discovery
Download file
PG-800
Size
800
compounds
Description
Phenyl Glutarimide (PG)-based library was purposefully developed to expand the structural diversity space of Molecular Glues
Download file
Len Library
Len-640
Size
640
compounds
Description
Lenalidomide- and Pomalidomide-scaffold based — a time-tested backbone, reimagined for modern Molecular Glue design
Download file
AAG Library
AAG-1600
Size
1 600
compounds
Description
Acylated Amino Glutarimide (AAG) featuring 5- and 6-membered heterocyclic systems: a promising platform for the discovery of next-generation Molecular Glues
Download file
Avadomide library
AVD-560
Size
560
compounds
Descriptions
A special selection of new Avadomide analogs
Download file
Diverse CRBN Library
CRBN-960
Size
960
compounds
Descriptions
Designed to cover all structural diversity of CRBN binders, a key component of the E3 ubiquitin ligase complex
Download file
CRBN Covalent Library
CCRBN-160
Size
160
compounds
Descriptions
A small selection of diverse covalent binders capable of interacting with CRBN
Download file
Representative examples of compounds in pre-plated CRBN Diversity Library

Examples of compounds in pre-plated CRBN Covalent Library

Examples of in-stock CRBN binders with linkers

Latest Arrivals
Glutarimide ring variation
Imide motif variation
Core structure variation
Designed to target DCAF family of E3 ligases
5 440 pre-plated compounds
For a long time, Indisulam and a few other clinically tested aryl sulfonamides such as Tasisulam and chloroquinoxaline sulfonamide (CQS) remained compounds with pronounced selective in vitro anticancer activity and unclear action mechanisms. Recent extensive investigations in cancer genome sequencing together with X-Ray crystallography and cryo-EM showed that these compounds act as a “molecular glue” to induce degradation of RBM39 via complex formation and recruiting CUL-4-DCAF15 E3 ligase. This insight provided structural and mechanistic data that significantly extended our understanding of their mode of action. Further research in this area reveals covalent binders of other members of DCAF family proteins, such as DCAF1, DCAF11, and DCAF16.
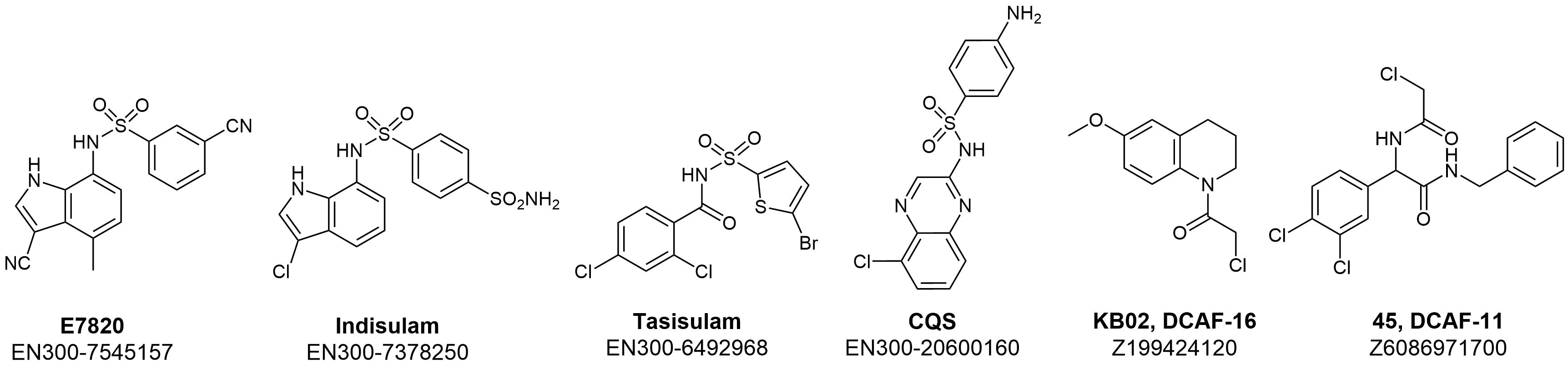
Recent structural data and reported DCAF binders allowed us to design a dedicated library of compounds with the potential to act as molecular glues. The library is now available in a pre-plated format and can be quickly delivered in any convenient for your project format.
Download SD file
Typical Formats
Catalog No.
DCAF Library
DCAF-5440-10-Y-10
Compounds
5 440
17 plates
Amount
10 µL of 10 mM DMSO solutions
Plates and formats
384-well Echo plates, Labcyte #LP-0200, 320 compounds per plate, first two and last two columns empty
Price
Catalog No.
DCAF Library
DCAF-5440-50-X-20
Compounds
5 440
68 plates
Amount
50 µL of 20 mM DMSO solutions
Plates and formats
96-well plates, 80 compounds per plate, first and last columns empty; Greiner #781270
Price
*We will be happy to provide our library in any other most convenient for your project format. Please select among the following our standard microplates: Greiner Bio-One 781270, 784201, 781280, 651201 or Echo Qualified 001-12782 (LP-0200), 001-14555 (PP-0200), 001-6969 (LP-0400), C52621 or send your preferred labware. Compounds pooling can be provided upon request.
Library Design
DCAF ligases have become more and more promising targets in protein degradation drug discovery. Proven efficacy and convenient chemistry make these targets attractive for developing new probes and drug candidates rapidly.
According to the structural data Indisulam and Tasisulam bind DCAF15 in an overall configuration similar to E7820, maintaining the backbone hydrogen bonds from the sulfonyl groups to DCAF15 Ala234 and Phe235 and the water-mediated hydrogen bonds. However, the methyl-to-hydrogen substitution at C4 in Indisulam limits the hydrophobic interactions with DCAF15 Val477 and Val556, while Tasisulam lacks the indole NH hydrogen bond to the backbone carbonyl of DCAF15 Phe231.
Based on the available structural data we have designed a library of potential SPLAMs from our stock Screening Collection using the following approach:
- Scaffold-based selection of potential DCAF binders using a set of known binders for DCAF15, DCAF16, DCAF11, and DCAF1.
- Generation and selection of close chemotypes/scaffolds based on diverse aryl and heteroaryl (five-membered inclusive) sulfonamides;
- Further selection based on pharmacophore modeling and docking results using available structural data of DCAF15;
- MedChem filtering according to criteria of lead/drug-likeness.
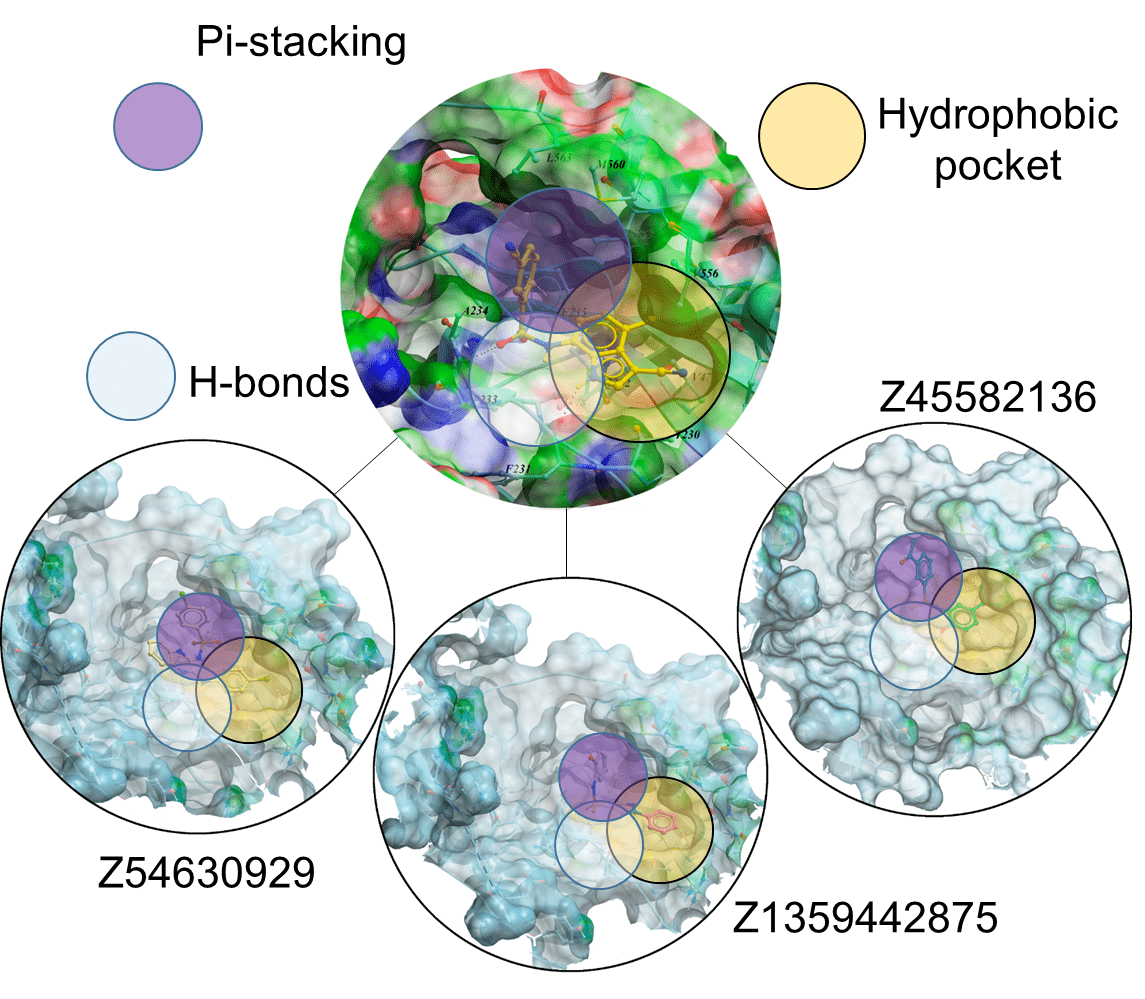
Docking results
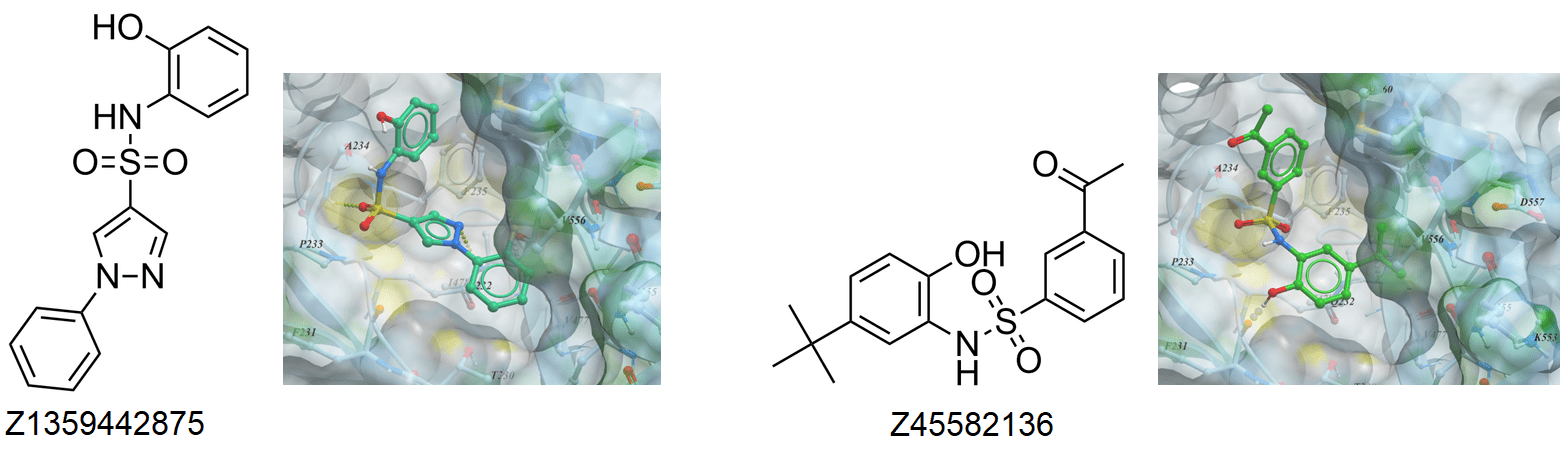
Examples of SPLAM analogues
Ready-to-use tools for your discovery
The recently emerged therapeutic area that modulates the ubiquitin-proteasome system needs new and reliable chemistry. Booming biological assays in this field of drug discovery require the production of hundreds and thousands of new functional intermediates, bifunctional molecules, and new molecular glues. The time needed to produce new derivatives is crucial at any stage of the drug discovery program. We offer advanced synthetic chemistry support, including custom library design and synthesis.
12500 compounds in stock and over 13.4 million in REAL
1360 compounds pre-plated Covalent Fragments Chloroacetamides Library
1200 compounds pre-plated Covalent Screening Chloroacetamides Library
320 compounds pre-plated Covalent Enantiomeric Pairs Fragments Chloroacetamides Library
Chloroacetamides are a well-known class of electrophilic warheads widely used in the design of covalent inhibitors, particularly for their ability to selectively bind to cysteine residues. Although chloroacetamides are often considered overly reactive, their main advantage over commonly used warheads such as acrylamides and vinyl sulfones, lies in their ability to form irreversible covalent bonds with target residues, enabling unambiguous detection of covalent binding events.
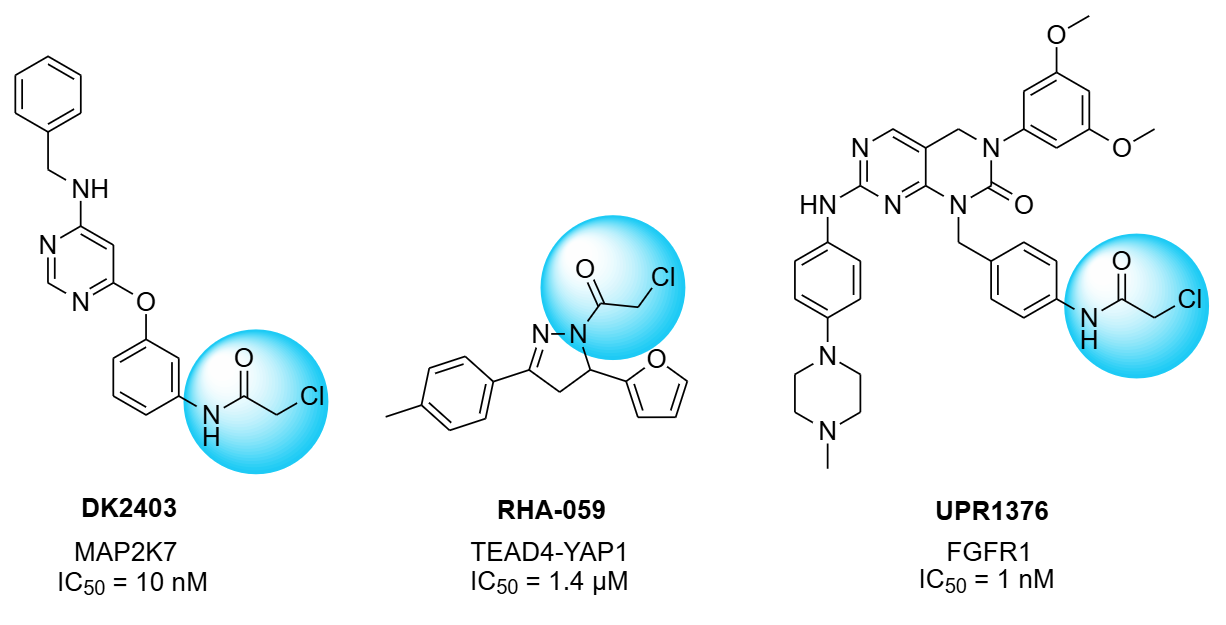
Several compounds with сhloroacetamide warheads are under active investigation and demonstrate encouraging therapeutic potential: RHA-059, UPR1376, and DK2403 (highly potent and selective covalent MAP2K7 inhibitor, targeting Cys218 in the kinase active site).
Enamine has almost 13k compounds in stock (90%+ pure by LCMS and/or NMR analysis), which can be delivered as dry powders or in any customized plated formats. Alternatively, you can acquire new compounds from our pool of more than 13.4 million REAL Сhloroacetamides. Synthesis takes only 3-4 weeks yielding over 80% of the selected compounds.
Download SD files
15 865 compounds for cherry-picking
Plated libraries in stock:
Examples of the chloroacetamides from stock
Chlorofluoroacetamides and 2-Chloropropionamides
3200 compounds in stock and over 12.4 million in REAL
320 compounds pre-plated Covalent Fragments Chlorofluoroacetamides Library
320 compounds pre-plated Covalent Fragments Chloropropionamides Library
560 compounds pre-plated Covalent Screening Chloropropionamides Library
Beyond chloroacetamides, chlorofluoroacetamides and chloropropionamides introduce unique reactivity profiles and molecular features, enhancing the versatility in warhead selection.
Сhlorofluoroacetamides exhibit reversible binding behavior, which provides an advantage in certain cases. For example, YH-6 is an aza-peptide inhibitor of SARS-CoV-2 3CLpro wich bonding Cys145.
2-Chloropropionamides are a less reactive class of electrophilic warheads compared to chloroacetamides, offering improved selectivity and tunability for covalent modification of target proteins. For example, S-CW3554 is selectively targets protein disulfide isomerase (PDIA1), demonstrating cytotoxicity in multiple myeloma cells.
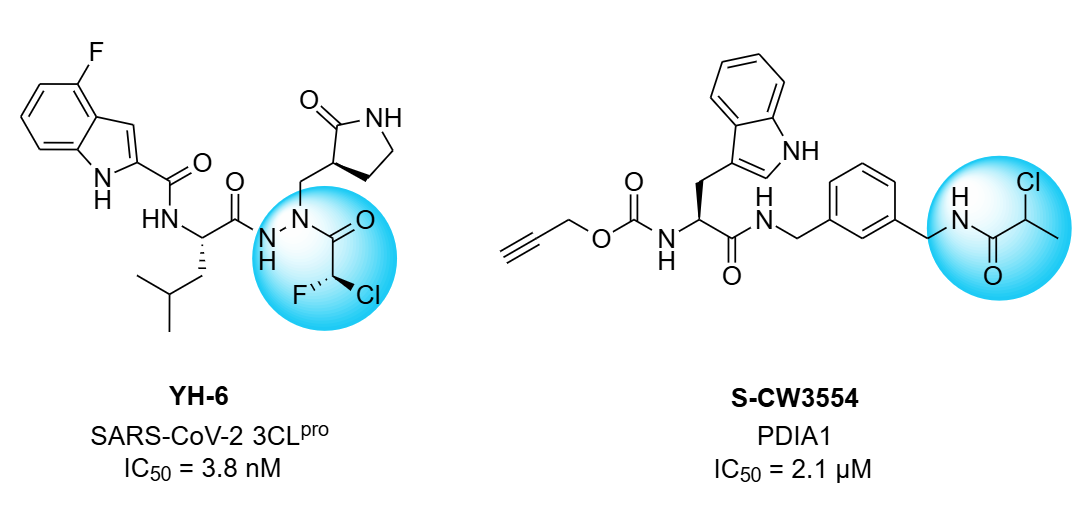
Examples of the chlorofluoroacetamides and chloropropionamides from stock
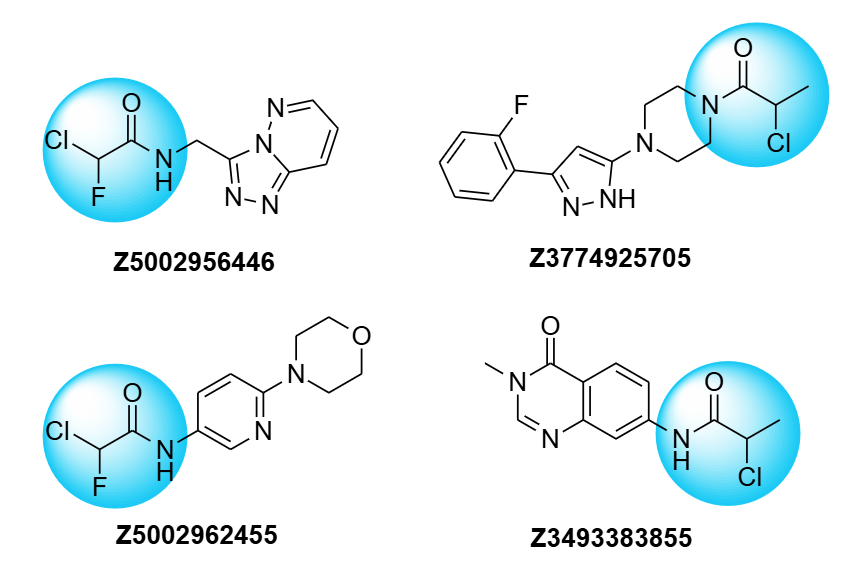
Enamine has 1.5k chlorofluoroacetamides and 1.7k chloropropionamides in stock (90%+ pure by LCMS and/or NMR analysis), which can be delivered as dry powders or in any customized plated formats.
The largest and most reliable source of novel covalent modifiers
153 430 compounds
Covalent screening has become an integral part of Drug Discovery and is now playing an essential role in hit finding, developing novel screening techniques, identifying new protein targets, and assessing their drugability.
We offer a wide range of covalent compound classes, from acrylamides and chloroacetamides to recently established niche classes whose stability and reactivity are yet to be studied. All compounds have been synthesized at Enamine using our in-house developed synthesis protocols and elaborated purification methods. This has allowed us to enumerate REAL Compound arrays – new covalent compounds that we can confidently synthesize within only 4 weeks.
We will be happy to help you with the covalentization of your scaffold, lead compound or simply give advice on covalent warhead attachment and its type.
Download SD files
Acrylamides
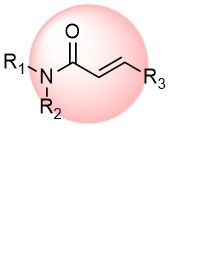
Acrylamides are one of the most popular classes of covalent warheads used for irreversible binding to non-catalytic cysteines of target proteins to enhance pharmacological potency and selectivity. We offer the most diverse collection of the terminal and substituted acrylamides, enabling the discovery of new covalent binders with fine-tuning reactivity and affinity to a target.
Over 42k AcrylamidesChloroacetamides
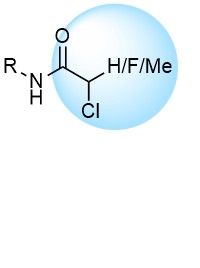
Chloroacetamides are commonly used to develop new covalent probes for challenging targets. Ease of adducts interpretation and high reactivity are their main advantages. Recently synthesized more stable and less reactive 2-chloropropionamides and chlorofluoroacetamides represent a high potential for further discoveries.
Over 15k ChloroacetamidesSulfonyl Fluorides
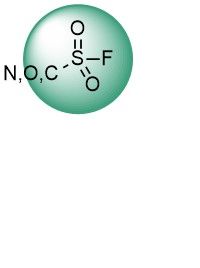
Sulfonyl fluorides have emerged as new, but already well-established, promising covalent binders targeting multiple nucleophilic amino acids such as Tyr, Lys, Ser, His, and Thr. Recent advances, including the application of SuFEx electrophiles in proteomics screens, reveal new horizons for this type of warhead. Their high stability and moderate reactivity allow the discovery of highly selective irreversible binders.
Over 6k Sulfonyl FluoridesBoronics and Formyl Boronates
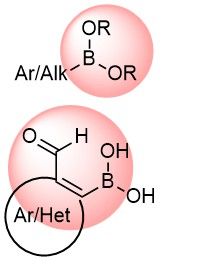
Boronic compounds occupy a special place in medicinal chemistry, including five FDA-approved drugs to date. Boron functional groups are considered covalent binders but are also often utilized in prodrugs, soft drugs, or as various carriers. We focused our efforts on the synthesis of new boronics that can be applied in the discovery of new selective Ser, Thr, and Lys reversible binders.
Over 8k Boronics and Formyl BoronatesEpoxides, Aziridines
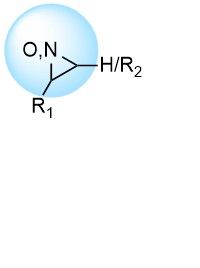
Epoxides and aziridines received relatively little attention, at the same time being among the first developed covalent drugs, including a number of nature-derived antibiotics such as fosfomycin.
Both epoxides and aziridines react irreversibly through SN2 mechanism with the formation of adducts, often with Cys residues.
Vinyl Sulfones
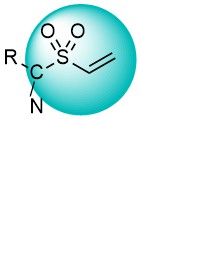
Vinyl sulfones and vinyl sulfonamides are active binders for Lys and Cys residues. They react faster than acrylamides and have shown some preference for binding Lys over Cys. These warheads have been widely used in the development of new selective inhibitors of kinases and other enzymes. An extensive set of versatile vinyl sulfonyl derivatives covering a wide reactivity range is available from Enamine.
Over 9k Vinyl SulfonesCyanoacrylamides
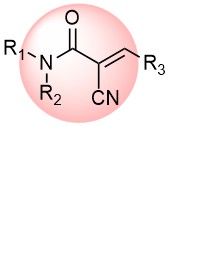
Cyanoacrylamide is a reversible covalent warhead acting on Cys residues. The introduction of a small CN group into α-position of acrylamide dramatically alters its properties, making it a distinct class of covalent binders. Most of them are more active than corresponding acrylamides, but activity can be fine-tuned by appropriate substitution. The presence of the motif in drugs supports its significance in the future of drug discovery.
Over 4k CyanoacrylamidesActivated Acetylenes
Activated acetylenes can irreversibly bind to cysteine residues. Selectivity, leading to fewer off-target interactions, could be achieved by tuning from acrylamide to propionamide derivatives. These warheads are well-validated and represented by approved drugs. Besides thousands of compounds in stock, we can quickly synthesize unique molecules on demand.
Aldehydes and Salicylic aldehydes
Aldehyde is a common chemical functionality, able to target both catalytic and noncatalytic cysteine residues by forming a reversible covalent bond. Also, they are able to target lysines by the formation of Schiff bases. Salicylic aldehydes are especially versatile in the latter case due to the enhanced stability of covalent adducts. Previously aldehydes were unpopular in drug discovery programs due to potential metabolic liability, but recent examples show surprisingly stable aldehydes passing clinical trials.
Cyanamides
Cyanamides (or N-Nitriles) are a versatile class of electrophilic warheads capable of forming reversible covalent bonds with nucleophilic residues, particularly cysteines, through thiourea adduct formation. Unlike more reactive electrophiles, cyanamides offer a balance between stability and reactivity, reducing the risk of off-target effects.
Over 700 CyanamidesDisulfides
Disulfide moiety can form a covalent bond with Cys residues by the thiol-disulfide exchange. Disulfide bridges are an important feature of cysteines in biological systems. Disulfide tethering is a useful technique used for the identification of hit fragments.
Strained Cyclobutanes
Bicyclo[1.1.0]butanes (BCB) recently emerged as a new class of thiol-reactive electrophiles and analogs of widely used acrylamides. Ring strain is utilized as a driving force for highly selective covalent tethering to nucleophilic amino acid residues. Hundreds of bicyclobutane carboxylic amides have been synthesized at Enamine and even more are to come from new syntheses
Over 500 Strained Cyclobutanes