Designed to target DCAF family of E3 ligases
5 440 pre-plated compounds
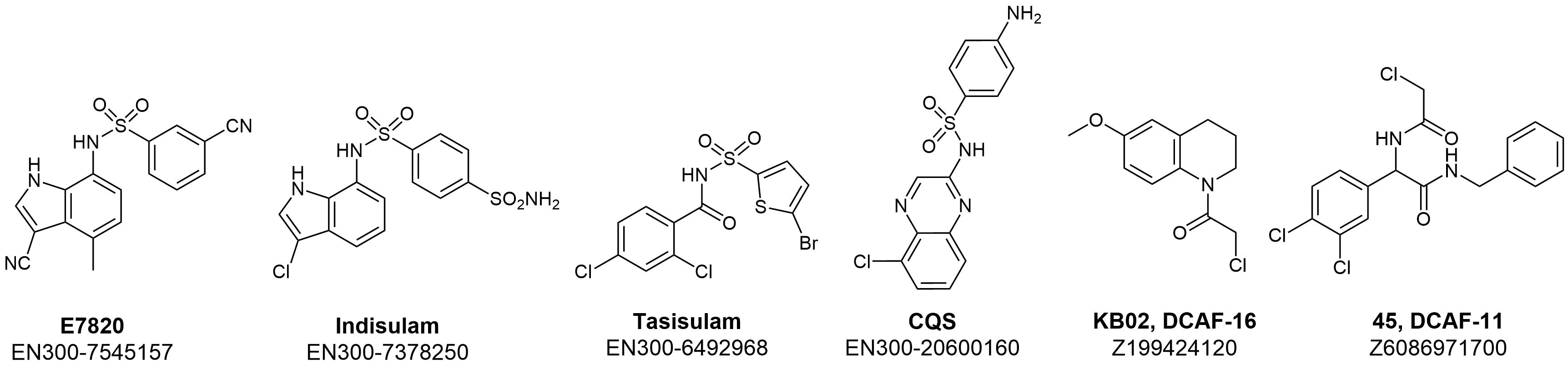
For a long time, Indisulam and a few other clinically tested aryl sulfonamides such as Tasisulam and chloroquinoxaline sulfonamide (CQS) remained compounds with pronounced selective in vitro anticancer activity and unclear action mechanisms. Recent extensive investigations in cancer genome sequencing together with X-Ray crystallography and cryo-EM showed that these compounds act as a “molecular glue” to induce degradation of RBM39 via complex formation and recruiting CUL-4-DCAF15 E3 ligase. This insight provided structural and mechanistic data that significantly extended our understanding of their mode of action. Further research in this area reveals covalent binders of other members of DCAF family proteins, such as DCAF1, DCAF11, and DCAF16.
Recent structural data and reported DCAF binders allowed us to design a dedicated library of compounds with the potential to act as molecular glues. The library is now available in a pre-plated format and can be quickly delivered in any convenient for your project format.
Download SD file
Typical Formats
Catalog No.
DCAF Library
DCAF-5440-10-Y-10
Compounds
5 440
17 plates
Amount
10 µL of 10 mM DMSO solutions
Plates and formats
384-well Echo plates, Labcyte #LP-0200, 320 compounds per plate, first two and last two columns empty
Price
Catalog No.
DCAF Library
DCAF-5440-50-X-20
Compounds
5 440
68 plates
Amount
50 µL of 20 mM DMSO solutions
Plates and formats
96-well plates, 80 compounds per plate, first and last columns empty; Greiner #781270
Price
*We will be happy to provide our library in any other most convenient for your project format. Please select among the following our standard microplates: Greiner Bio-One 781270, 784201, 781280, 651201 or Echo Qualified 001-12782 (LP-0200), 001-14555 (PP-0200), 001-6969 (LP-0400), C52621 or send your preferred labware. Compounds pooling can be provided upon request.
Library Design
DCAF ligases have become more and more promising targets in protein degradation drug discovery. Proven efficacy and convenient chemistry make these targets attractive for developing new probes and drug candidates rapidly.
According to the structural data Indisulam and Tasisulam bind DCAF15 in an overall configuration similar to E7820, maintaining the backbone hydrogen bonds from the sulfonyl groups to DCAF15 Ala234 and Phe235 and the water-mediated hydrogen bonds. However, the methyl-to-hydrogen substitution at C4 in Indisulam limits the hydrophobic interactions with DCAF15 Val477 and Val556, while Tasisulam lacks the indole NH hydrogen bond to the backbone carbonyl of DCAF15 Phe231.
Based on the available structural data we have designed a library of potential SPLAMs from our stock Screening Collection using the following approach:
- Scaffold-based selection of potential DCAF binders using a set of known binders for DCAF15, DCAF16, DCAF11, and DCAF1.
- Generation and selection of close chemotypes/scaffolds based on diverse aryl and heteroaryl (five-membered inclusive) sulfonamides;
- Further selection based on pharmacophore modeling and docking results using available structural data of DCAF15;
- MedChem filtering according to criteria of lead/drug-likeness.
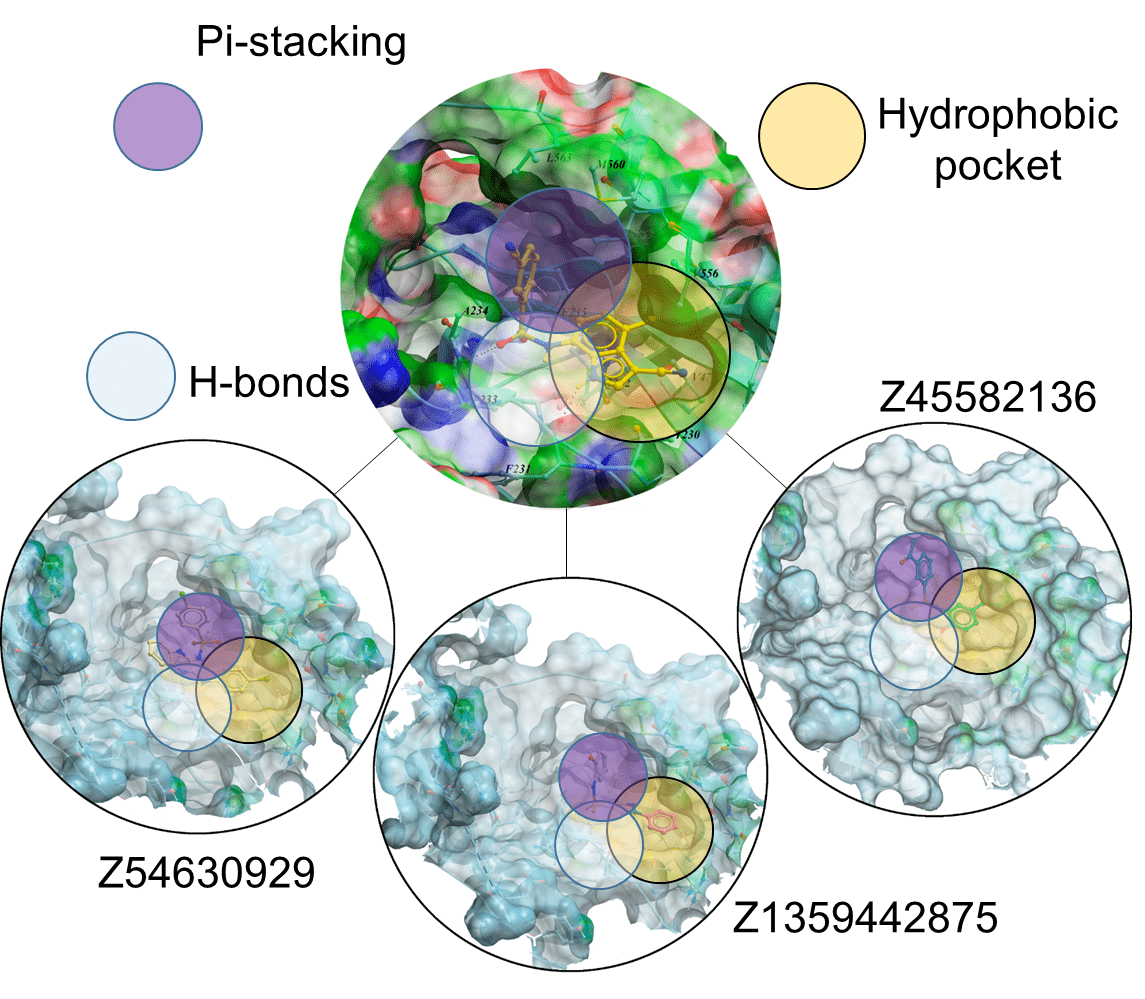
Docking results
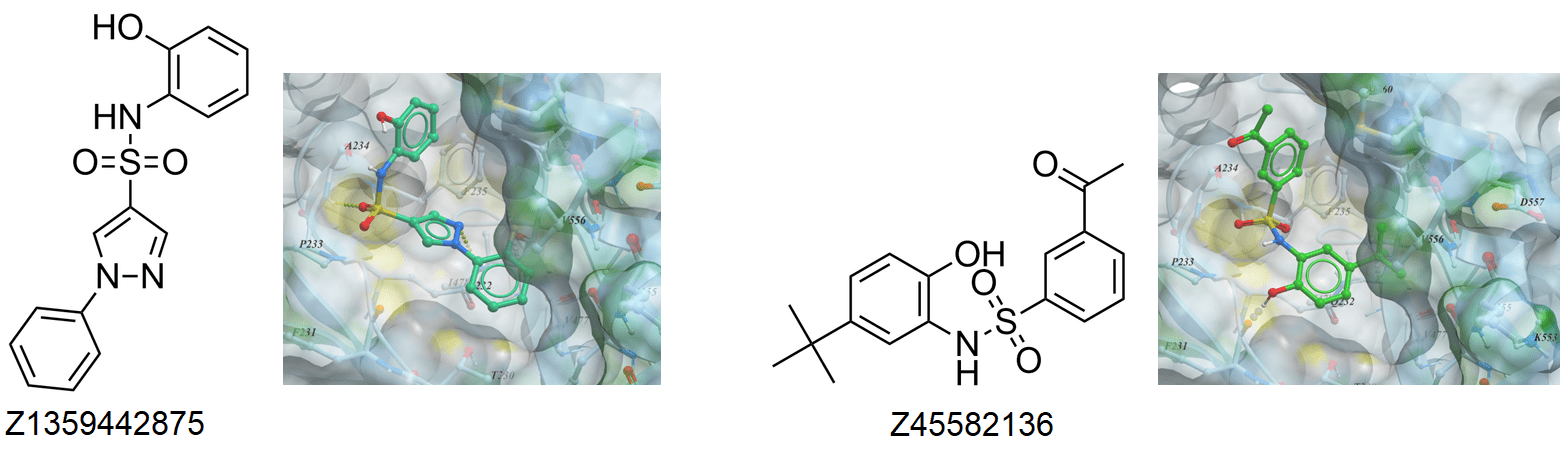
Examples of SPLAM analogues



