Product Focus
Amino acids are the building blocks which are widespread in the Nature. Even of the limited number of the genetically-coded α-amino acids the Nature composes practically unlimited number of proteins. The “success” of proteins in sustaining life and regulating biochemical processes prompted chemists over the world to mimic their structure and function in search for new drug candidates – peptidomimetics. It is therefore not surprising that the selection of the natural proteinogenic amino acids was substantially enriched by creating new, unnatural amino acids, specially designed to improve pharmacokinetic and pharmacodynamic properties of the peptidomimetics and other biologically active compounds based on them.
"The α-amino acids, molecules of which have restricted conformational flexibility, are widely used in design of peptidomimetics, peptide models, and in systematic search for biologically active compounds. Among these amino acids, a distinct class of compounds can be highlighted, namely - conformationally rigid amino acids (CRA). Certain torsion angles, which describe the conformation of a polypeptide chain at the CRA, are "fixed" that allows predicting and controlling it to some extent. Many structural studies show that the CRA residues can dictate certain conformation of the peptide chain around them, consequently, they can stabilize or destabilize certain peptide secondary structure elements.
For Palladium-Catalysed C–N And C–C Cross-Couplings.
Air and moisture insensitive auxiliary ligands for highly efficient palladium catalysts. These ultra-low loading (high TONs or TOFs) catalysts have shown excellent activity in Suzuki cross-coupling with aryl bromides and chlorides, Heck vinylation, Heck-Sonogashira alkynylation and allylic amination of allyl acetates (Tsuji–Trost type reactions). Low loadings of the catalysts ensure minimal contamination of the final compounds with palladium and simplify the purification procedures. High stability of the allows to store them for unlimited time without special precautions.
Recently 3-amino-tetrahydrothiophen-1,1-dioxides have drawn significant attention of a major drug developers. A few publications have been released earlier, corroborating rather high potential of this pharmacophoric moiety.
In the last decade, development of new drugs increasingly requires the use of chiral building blocks for hit-to-lead optimization, and even on early stages - in search for efficient hit compounds. The main fundamental reason for this lies in the fact that almost all the biological targets are chiral, and the drug-receptor interaction requires strict match of chirality. The formal reason results from strengthening the regulatory guidance for submitting new drug applications in Europe and USA which concern chirality issues.
We synthesize chiral ligands for metal-based catalysts used in asymmetric synthesis:
• Chiral chelating diphosphines, phosphine-phosphinites, both known and specially designed for your purpose. These ligands are used in Rhodium or Ruthenium complexes capable to catalyse asymmetric hydrogenation of prochiral C=C and C=O bonds, hydrosilylation, hydroformylation (see, for example: Asymmetric Synthesis, (Ed.: J. D. Morrison), Academic Press, Orlando, 1985; R. Noyori, Asymmetric Catalysis in Organic Synthesis, Wiley, New York, 1994; Comprehensive Asymmetric Catalysis, (Eds.: E. N. Jacobsen, A. Pfaltz, H. Yamamoto), Springer, Heidelberg, 1999, Vol. I-III. Catalytic Asymmetric Synthesis, (Ed.: I. Ojima), Wiley-VCH, New York, 2000).
Enamine Company would like to present new Building Blocks containing CONH2 or NHCONH2 groups in side chains. These compounds also bear other functional groups allowing easy transformations to desired screening compounds with primary amide and ureide moieties.
Quite often hydrogen bonding interaction of a ligand amide group with a protein has its functional consequences. To a great extend protein - ligand binding occurs via hydrogen bonding. In the search for new hits and leads it is very important in combinatorial synthesis to use the least possible number of synthetic steps. Reactions of sulfonic acid chlorides are well studied and quite often used in nucleophilic substitution reaction.
Current reagent is an effective and facile generator of difluorocarbene. Phenyl(trifluoromethyl)mercury is a stable, crystalline solid which releases CF2 under mild, nonbasic reaction conditions. The compound is hydrolytically stable and can be handled under standard laboratory conditions. The reaction of PhHgCF3 and 3 molar equiv of anhydrous sodium iodide in benzene medium in the presence of olefins serves excellently in the synthesis of gem-difluorocyclopropanes. High products yields could be obtained in reaction times of about 15hr at 80-85°C.
Piperazines are essential elements in drug design. Currently MDL© Drug Data Report database contains over 11 800 structures bearing the piperazine moiety. Enamine would like to present our set of exclusive "small" piperazines - compounds with low molecular weight (below 190 Da).
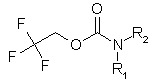
Enamine Company presents new building blocks to our valued customers. They can be used as good precursors for the preparation of unsymmetrical ureas. We have developed and synthesized a set of compounds of general formula:
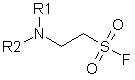
A new type of hydrolytically stable aliphatic sulfonyl fluorides matching general formula were developed and synthesized in our Company.