For Palladium-Catalysed C–N And C–C Cross-Couplings.
Air and moisture insensitive auxiliary ligands for highly efficient palladium catalysts. These ultra-low loading (high TONs or TOFs) catalysts have shown excellent activity in Suzuki cross-coupling with aryl bromides and chlorides, Heck vinylation, Heck-Sonogashira alkynylation and allylic amination of allyl acetates (Tsuji–Trost type reactions). Low loadings of the catalysts ensure minimal contamination of the final compounds with palladium and simplify the purification procedures. High stability of the allows to store them for unlimited time without special precautions.
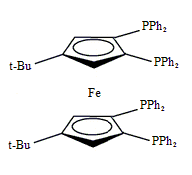
Tetraphos Fc(P)4
EN400-15231
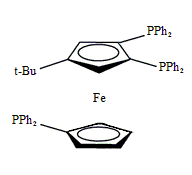
Triphos Fc(P)3
EN400-15232
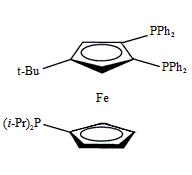
Triphos Fc(P)2PiPr
EN400-15233
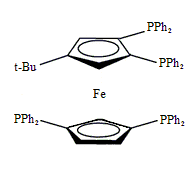
Tetraphos Fc(P)2(P’)2
EN400-15234
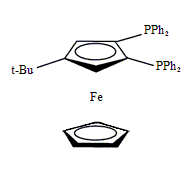
Diphos Fc(P)2
EN400-15235
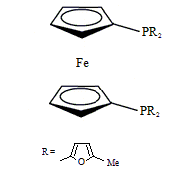
Diphos Fc(PFuMe)2
EN400-15236
Ligands are available in milligram and multigram quantities.
Download this article
EXAMPLES & NOTES:
(J.-C. Hierso et al. Organometallics (2003), 22(22), 4490-4499.)
Suzuki cross-coupling with aryl bromides at ultra-low catalyst loadings (L = Tetraphos Fc(P)4 EN400-15231)
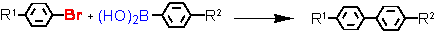
R1
C(=O)Me
R2
H
Ratio S/Cat.
100 000
Yield, %
100 (94)a
R1
CN
R2
H
Ratio S/Cat.
100 000
Yield, %
100 (87)a
R1
CF3
R2
H
Ratio S/Cat.
100 000
Yield, %
100
R1
OMe
R2
H
Ratio S/Cat.
100 000
Yield, %
77
R1
OMe
R2
H
Ratio S/Cat.
10 000
Yield, %
100 (92)a
a - isolated yield, conditions: 1 Pd / 1 Tetraphos Fc(P)4: 10-2 - 10-4 mol%; DMF or xylene, K2CO3, 130 °C, 20h.
Suzuki cross-coupling with aryl chlorides (L = Tetraphos Fc(P)4 EN400-15231)
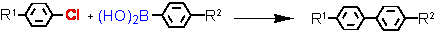
R1
C(=O)Me
R2
OMe
Ratio S/Cat.
1000
Yield, %
98 (88)a
R1
C(=O)Me
R2
OMe
Ratio S/Cat.
10 000
Yield, %
74
R1
CN
R2
OMe
Ratio S/Cat.
1000
Yield, %
89 (83)a
a - isolated yield, conditions: 1 Pd / 1 Tetraphos Fc(P)4 : 10-1 - 10-2 mol%; DMF or xylene, K2CO3, 130 °C, 20h.
Heck reaction at ultralow catalyst loadings (L = Tetraphos Fc(P)4 EN400-15231)
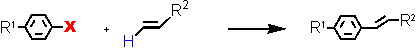
Aryl Halide
PhI
Alkene
n-BuOCOCH=CH2
Ratio S/Cat.
1000 000
Yield, %
100 (85)a
Time, h
48
Aryl Halide
4-MeOC6H4Br
Alkene
n-BuOCOCH=CH2
Ratio S/Cat.
10 000
Yield, %
100 (88)a
Time, h
48
Aryl Halide
4-MeOC6H4Br
Alkene
C6H5CH=CH2
Ratio S/Cat.
100 000
Yield, %
65a
Time, h
20
Aryl Halide
4-CH(O)C6H4Br
Alkene
n-BuOCH=CH2
Ratio S/Cat.
250
Yield, %
100 (83)a
Time, h
20
Aryl Halide
4-CH(O)C6H4Br
Alkene
n-BuOCH=CH2
Ratio S/Cat.
1000
Yield, %
41a
Time, h
20
a - isolated yield, conditions: 1 Pd / 1 Tetraphos Fc(P)4, Xylene, K2CO3, 130 °C.
Alkynylation of aryl halides (Heck-Sonogashira reaction) under low (10-1 to 10-4 mol%) catalyst loadings in the presence of a Triphos Fc(P)2PiPr EN400-15233 with TONs up to 250000. Copper-free coupling using phenyl- acetylene is also accessible in good yield.
J.-C. Hierso, V. Ivanov et al., Organic Letters (2004), 6(20), 3473-3476.
H. Doucet, J.-C. Hierso, Angewandte Chemie International Edition (2007), 46, 834-871.
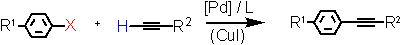
Aryl Halide
PhI
Alkene
phenylacetylene
Ratio S/Cat.
100 000
Yield, %
95
Aryl Halide
4-Bromoacetophenone
Alkene
phenylacetylene
Ratio S/Cat.
100 000
Yield, %
94
Aryl Halide
4-Bromobenzonitrile
Alkene
phenylacetylene
Ratio S/Cat.
10 000
Yield, %
89
Aryl Halide
4-Chlorobenzonitrile
Alkene
phenylacetylene
Ratio S/Cat.
250
Yield, %
86a
Aryl Halide
4-Bromoanisole
Alkene
phenylacetylene
Ratio S/Cat.
250
Yield, %
93
Aryl Halide
4-Bromoacetophenone
Alkene
but-1-yn-4-ol
Ratio S/Cat.
250
Yield, %
92b
aReaction performed without CuI. bReaction performed at 100 °C / Conditions: catalyst {Pd(C3H5)Cl}2/Triphos Fc(P)2Pi-Pr 1/2, aryl halide (1 mmol), alkyne (2 mmol), CuI (0.05 mmol), K2CO3 (2 mmol), DMF, 130 °C, 20 h, isolated yields.
Selective Amination of allylic acetates under low catalyst loadings in the presence of ferrocenyldiphosphine Diphos Fc(PFuMe)2 EN400-15236
J.-C. Hierso et al. Advanced Synthesis and Catalysis (2005), 347(9), 1198-1202.
J.-C. Hierso, H. Doucet et al. Tetrahedron (2005), 61, 9759-9766.

Acetate
Allyl acetate
Amine
aniline
Ratio S/Cat.
100
Conditions
Rt, 1h
Conversion, %
100
Selectivity
96/4
TOF (h-1)
10 000
Acetate
Allyl acetate
Amine
piperidine
Ratio S/Cat.
10 000
Conditions
Rt, 1h
Conversion, %
100
Selectivity
-
TOF (h-1)
5 000
Acetate
Allyl acetate
Amine
morpholine
Ratio S/Cat.
10 000
Conditions
Rt, 2h
Conversion, %
85
Selectivity
100
TOF (h-1)
4 250
Acetate
Allyl acetate
Amine
diisopropylamine
Ratio S/Cat.
1 000
Conditions
80 °C 2h
Conversion, %
96
Selectivity
100
TOF (h-1)
480
Acetate
Hex-2-en-1-yl acetate
Amine
pyrrolidine
Ratio S/Cat.
1 000
Conditions
50 °C, 20h
Conversion, %
100
Selectivity
93/7(lin/br)
TOF (h-1)
-
Acetate
Hex-2-en-1-yl acetate
Amine
morpholine
Ratio S/Cat.
1 000
Conditions
50 °C, 20h
Conversion, %
98
Selectivity
94/6(lin/br)
TOF (h-1)
-
Acetate
Cinnamyl acetate
Amine
pyrrolidine
Ratio S/Cat.
1 000
Conditions
50 °C, 20h
Conversion, %
100
Selectivity
94/6(lin/br)
TOF (h-1)
2 600
Acetate
Cinnamyl acetate
Amine
morpholine
Ratio S/Cat.
1 000
Conditions
50 °C, 20h
Conversion, %
100
Selectivity
93/7(lin/br)
TOF (h-1)
4 800
Acetate
Cinnamyl acetate
Amine
diethylamine
Ratio S/Cat.
1 000
Conditions
Rt, 20h
Conversion, %
100
Selectivity
94/6(lin/br)
TOF (h-1)
7 600
Acetate
Geranyl acetate
Amine
morpholine
Ratio S/Cat.
1 000
Conditions
80 °C, 3h
Conversion, %
100
Selectivity
98
TOF (h-1)
33
Catalyst {Pd(C3H5)Cl}2 / Diphos Fc(PFuMe)2 Conditions: S/C = 250-10000, T = 25°C-50 °C, 2 equiv. amine, 1 to 20 h.
