We synthesize chiral ligands for metal-based catalysts used in asymmetric synthesis:
• Chiral chelating diphosphines, phosphine-phosphinites, both known and specially designed for your purpose. These ligands are used in Rhodium or Ruthenium complexes capable to catalyse asymmetric hydrogenation of prochiral C=C and C=O bonds, hydrosilylation, hydroformylation (see, for example: Asymmetric Synthesis, (Ed.: J. D. Morrison), Academic Press, Orlando, 1985; R. Noyori, Asymmetric Catalysis in Organic Synthesis, Wiley, New York, 1994; Comprehensive Asymmetric Catalysis, (Eds.: E. N. Jacobsen, A. Pfaltz, H. Yamamoto), Springer, Heidelberg, 1999, Vol. I-III. Catalytic Asymmetric Synthesis, (Ed.: I. Ojima), Wiley-VCH, New York, 2000).
New original ligands are based on chiral natural terpenoids, mostly on camphor. Some examples of the ligands we can synthesize are shown below:
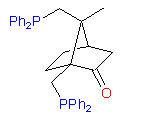
1
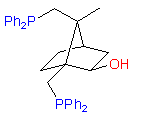
2
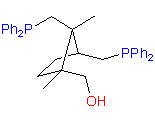
catASium I©
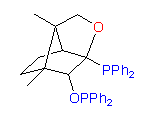
3
The hydroxydiphosphine ligand catASium I© and phosphine-phosphinite 3 are produced in collaboration with Degussa (patent application WO 03/099532). The compounds can be used for research purposes only and commercial quantities (5 g to several kg) are available from Degussa only (www.degussa-catalysts.com).
All the ligands are of >90% chemical purity and >99% optical purity.
• Chiral aminoalcohols used as chiral auxiliaries or as ligands for asymmetric catalysts (see Comprehensive Asymmetric Catalysis, (Eds.: E. N. Jacobsen, A. Pfaltz, H. Yamamoto), Springer, Heidelberg, 1999, Vol. I-III). We develop syntheses of new aminoalcohols starting from chiral pool, examples are shown below:
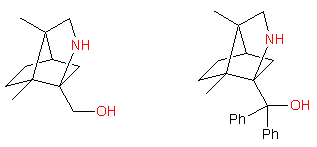
The aminoalcohols are of >95% chemical purity and >99% optical purity. Up to 2 g of each compound could be synthesized.
