In the last decade, development of new drugs increasingly requires the use of chiral building blocks for hit-to-lead optimization, and even on early stages - in search for efficient hit compounds. The main fundamental reason for this lies in the fact that almost all the biological targets are chiral, and the drug-receptor interaction requires strict match of chirality. The formal reason results from strengthening the regulatory guidance for submitting new drug applications in Europe and USA which concern chirality issues.
The following citation of the FDA’s “Policy Statement for the Development of new Stereoisomeric Drugs” illustrates this point: “Now that technological advances (large scale chiral separation procedures or asymmetric syntheses) permit production of many single enantiomers on a commercial scale, it is appropriate to consider what FDA's policy with respect to stereoisomeric mixtures should be… Where little difference is observed in activity and disposition of the enantiomers, racemates may be developed. In some situations, development of a single enantiomer is particularly desirable (e.g., where one enantiomer has a toxic or undesirable pharmacologic effect and the other does not).”
Therefore, it is not surprising that the market share of single isomer drugs rose to appreciable 35% by the year 2000 (Fig. 1), and continues to increase nowadays.
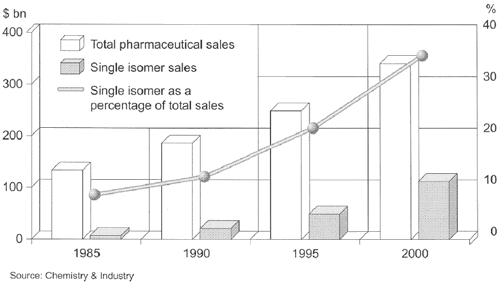
Fig. 1. Total and single isomer pharmaceutical sales.
Chiral optically pure building blocks have long been considered as less available than non-chiral – hence the widespread common opinion that non-chiral building blocks are more preferable than chiral, especially on early stages of drug development. Enamine would like to refute this opinion by offering a large selection of easily available, optically pure, functionalized chiral building blocks.
The Enamine’s chemists use all the general approaches to obtaining the chiral building blocks – chiral pool compound modification, asymmetric synthesis (including asymmetric catalysis), and large-scale enantioseparation. The latter became possible due to collaboration between Enamine and SiChem, the expert in chromatographic separation of enantiomers on multigram scale. Resolution of enantiomers by crystallization with chiral optically active resolution agents is also used.
All the synthesized chiral building blocks are analysed at Enamine for optical purity using modern chromatographic, NMR and polarimetric techniques. In particular, chromatographic determination of optical purity is possible on Agilent 1100 chromatograph equipped with ChiraDex and Chiralpack chiral stationary phase analytical columns. NMR analysis of chiral amines and alcohols is done using Mosher’s reagent, the carboxylic acids are analysed by NMR in the presence of chiral additives, and finally, many functionalized compounds are analysed with the help of NMR shift reagents.
We also can synthesize chiral building blocks corresponding to your needs using resolution of racemates (by chromatography or crystallization with resolution agents) or by various stereoselective synthetic approaches.
Optically pure compounds of different classes are available (see the examples below for best-sellers and representative examples). The compounds are ether in stock or can be prepared in agreed amounts in 2-3 weeks.
Natural amino acid derivatives (source of chirality – chiral pool)
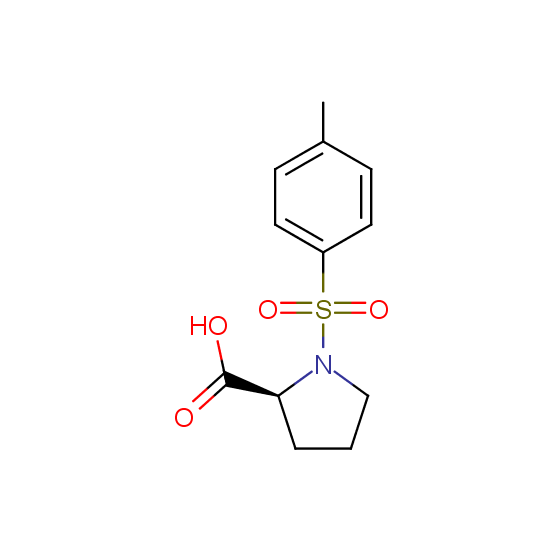
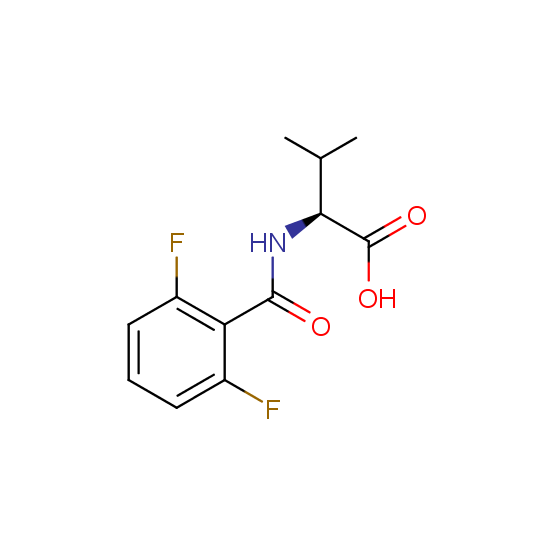
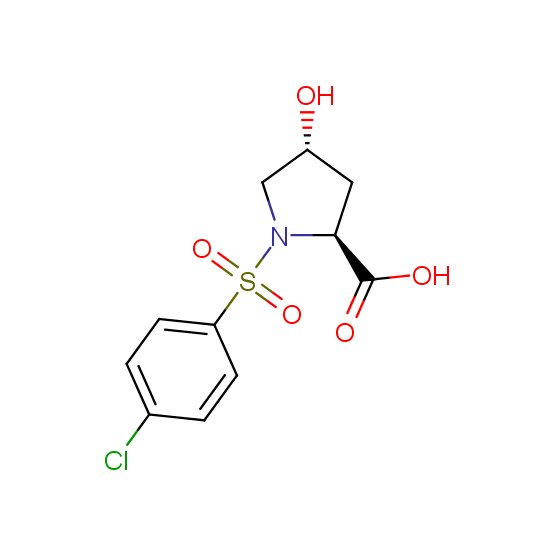
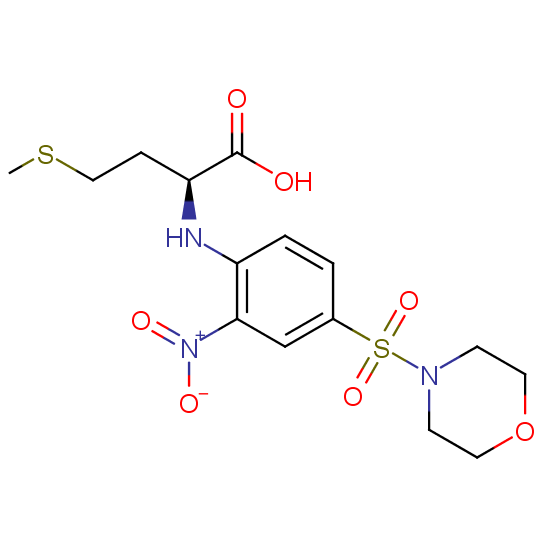
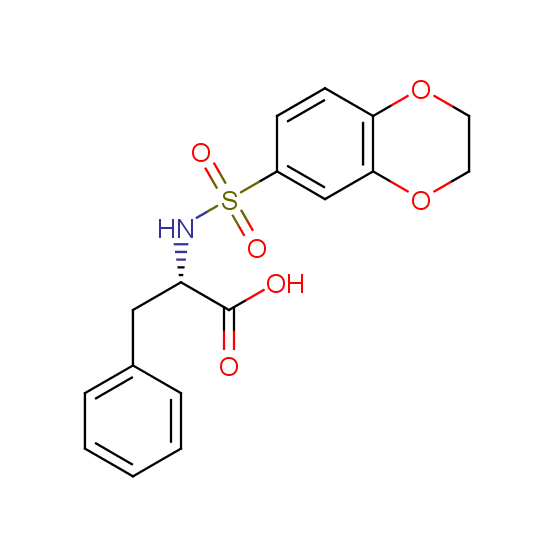
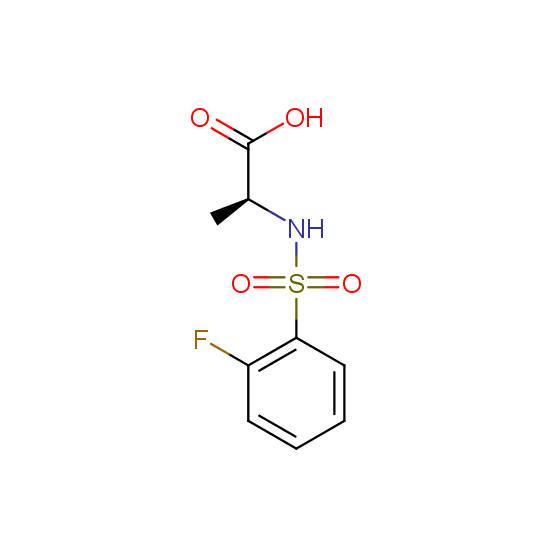
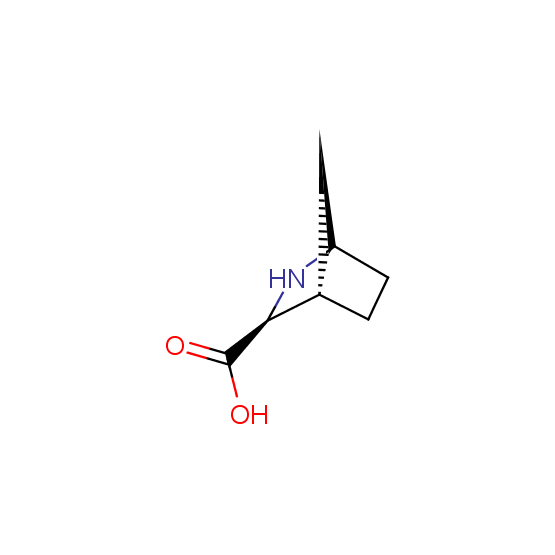
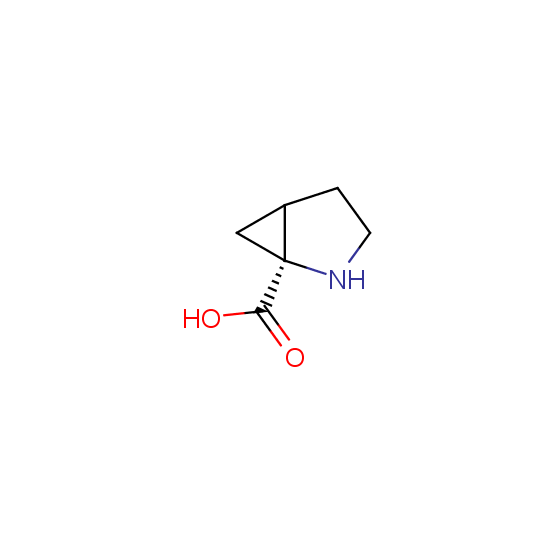
Unnatural amino acids (source of chirality – separation of diastereomers, chiral pool, separation of enantiomers)
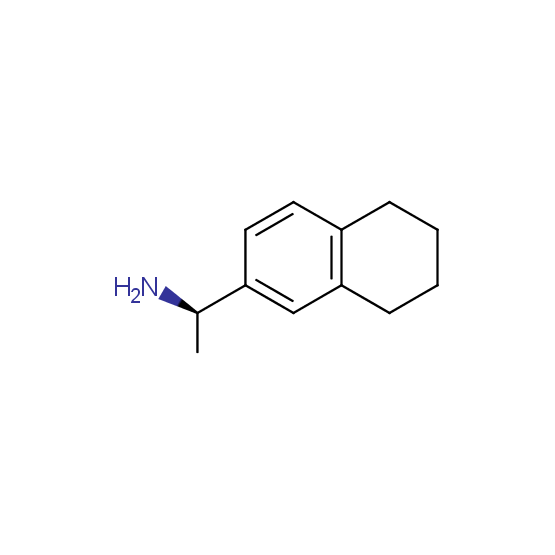
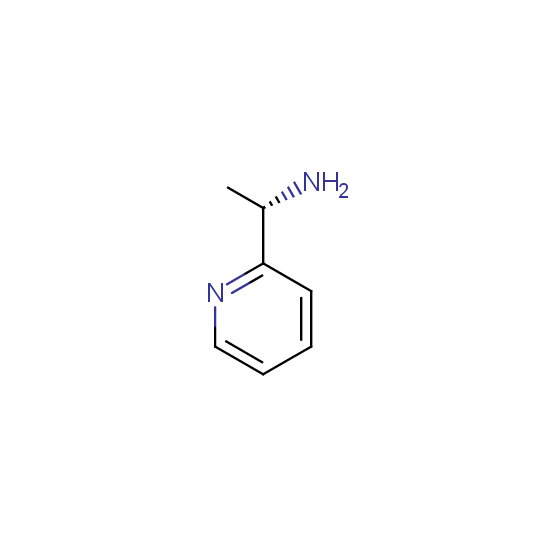
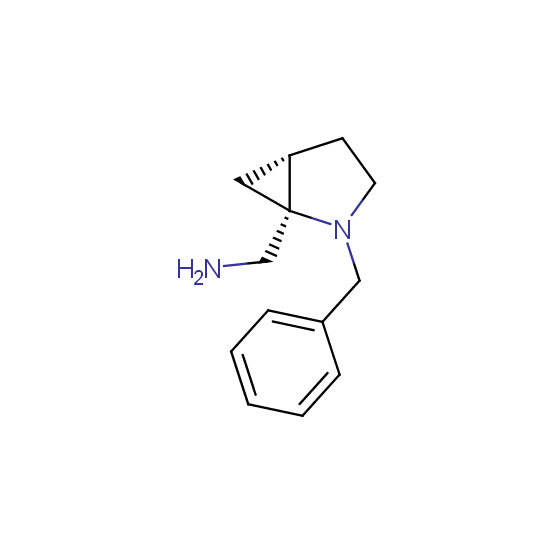
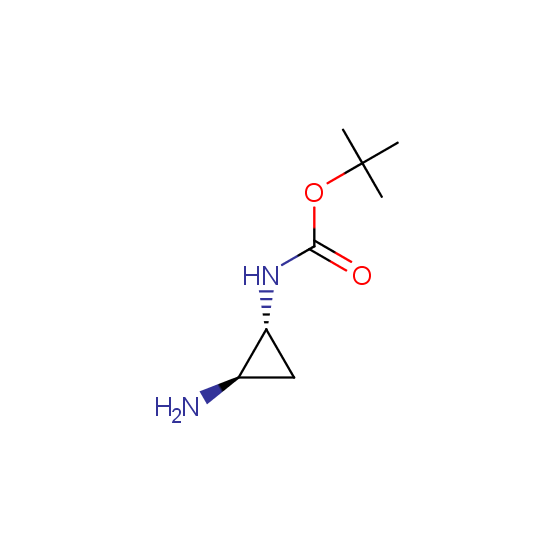
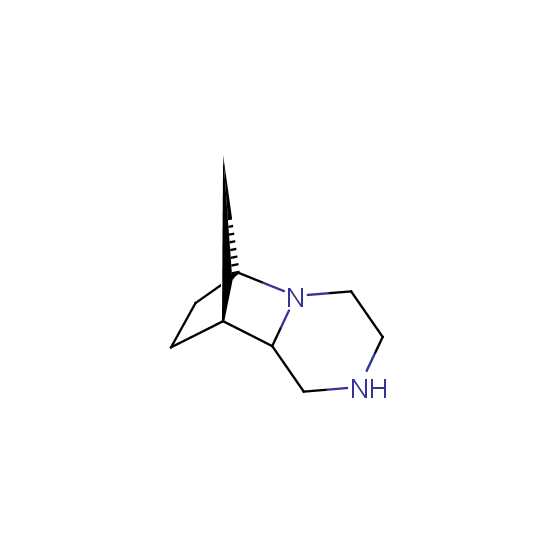
Diamines (including monoprotected) (source of chirality asymmetric synthesis, chiral pool)
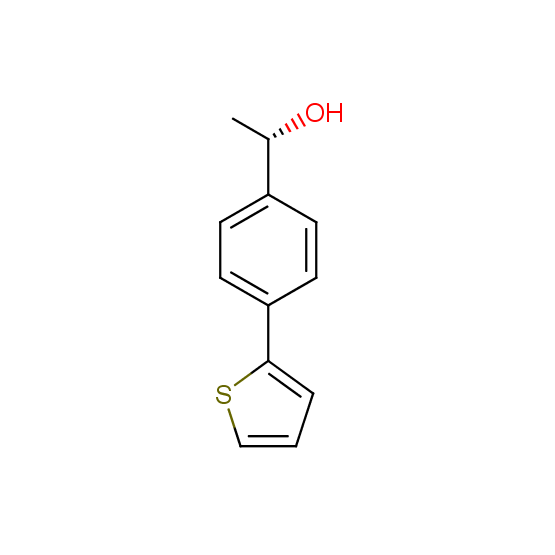
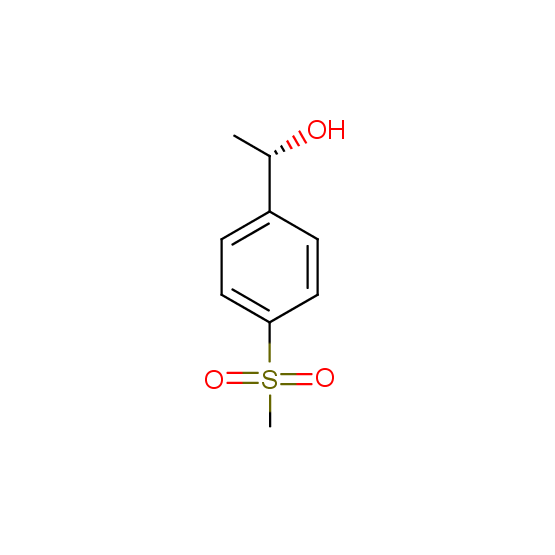
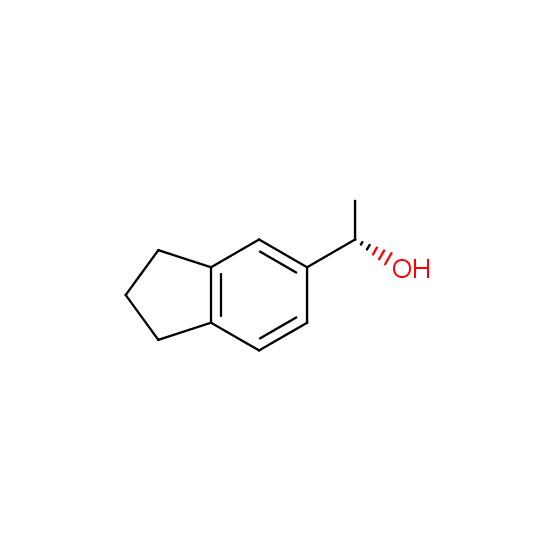
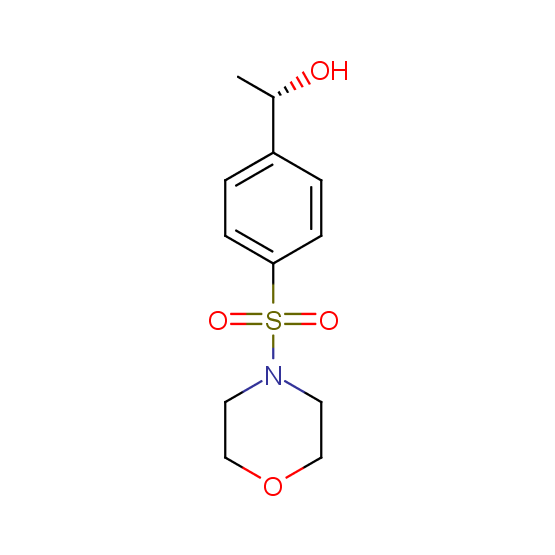
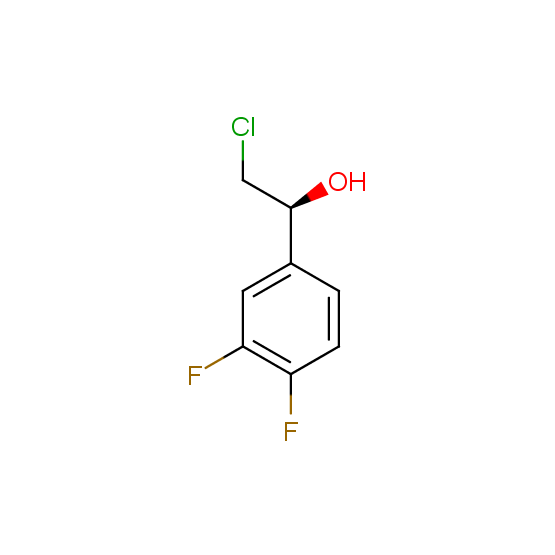
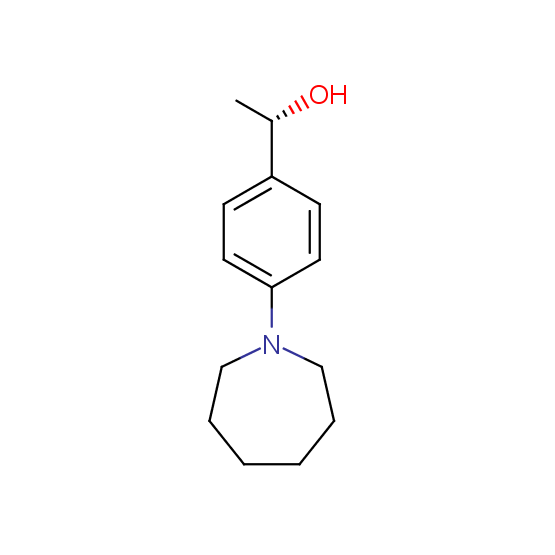
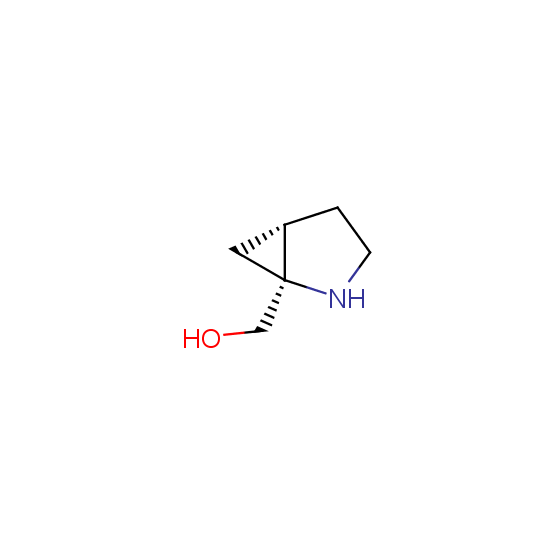
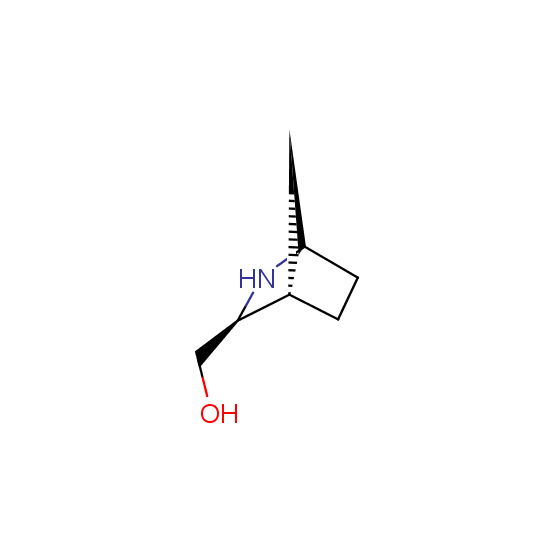
Alcohols (source of chirality – catalytic asymmetric synthesis and enantioseparation)
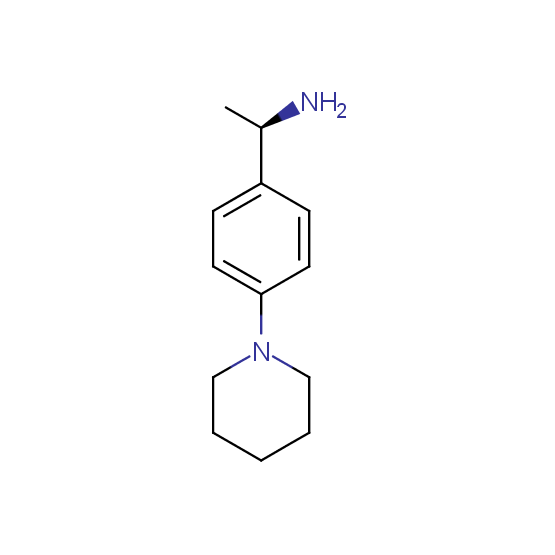
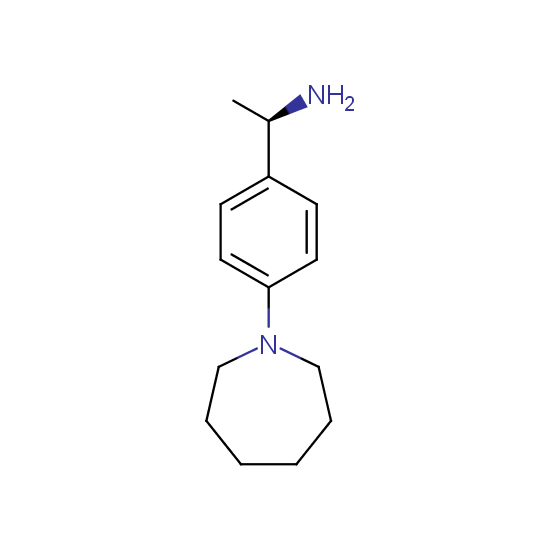
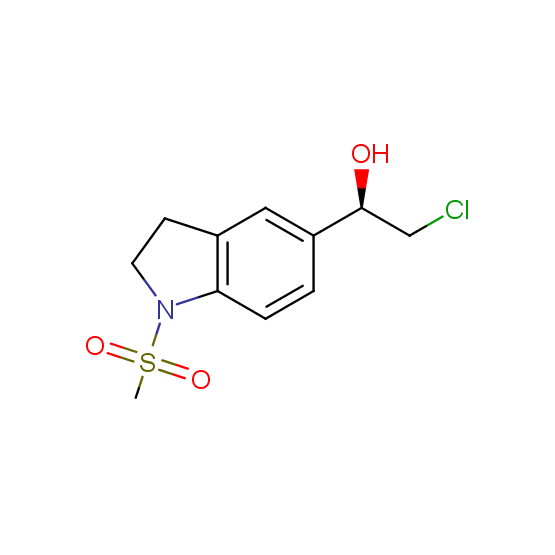
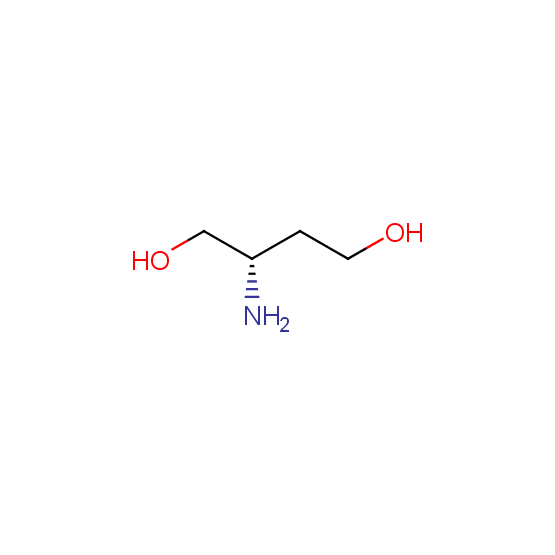
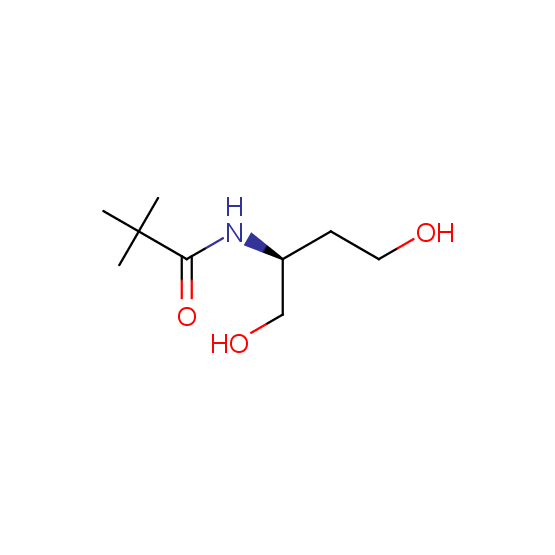
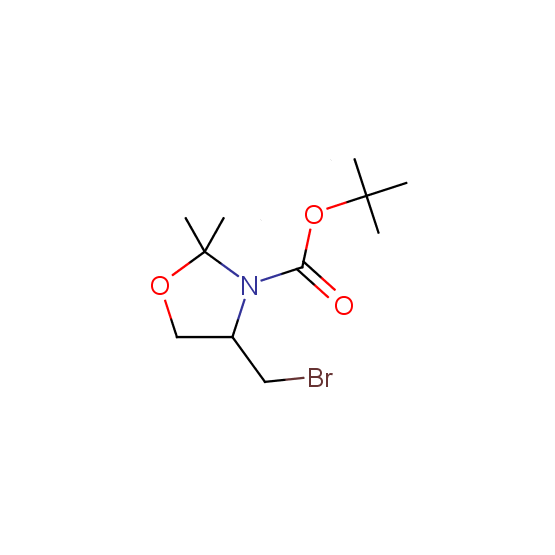
Aminoalcohols, including N- or/and O-protected (source of chirality – asymmetric synthesis and enantioseparation)
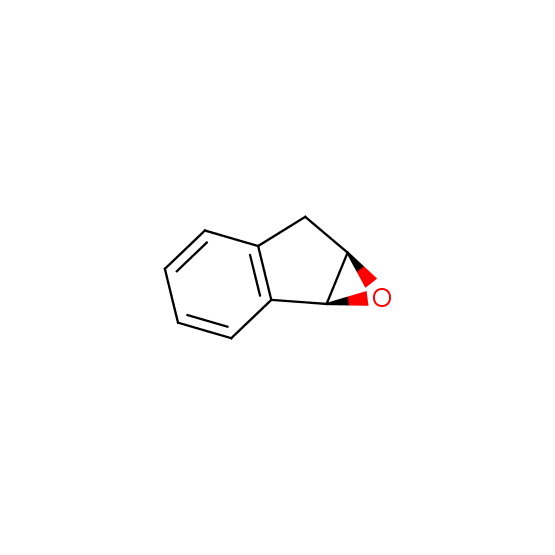
Epoxides (source of chirality – catalytic asymmetric synthesis)
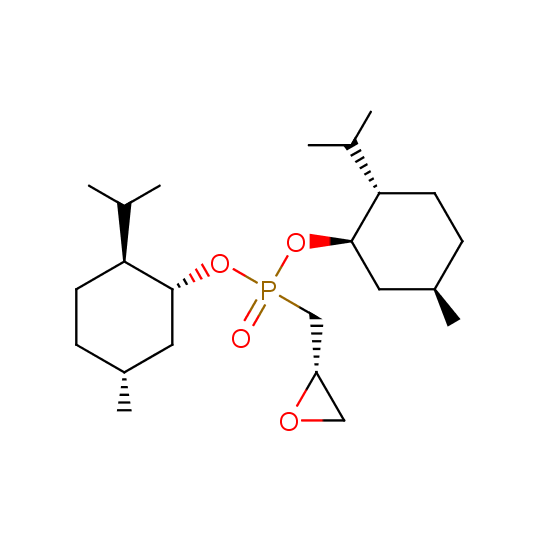
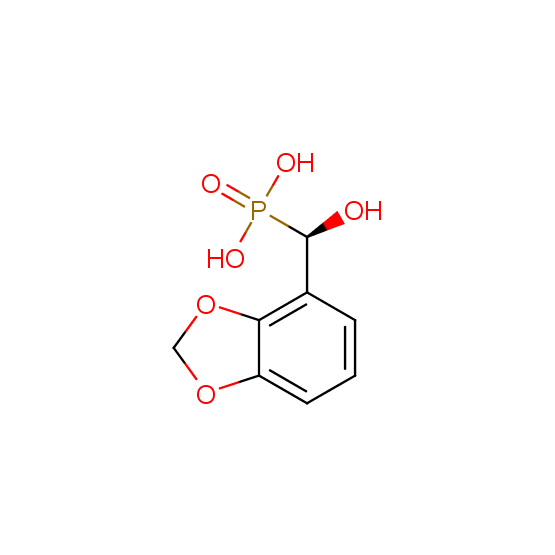
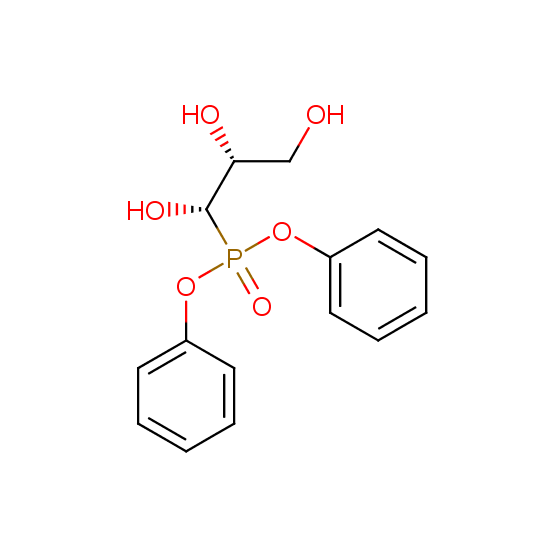
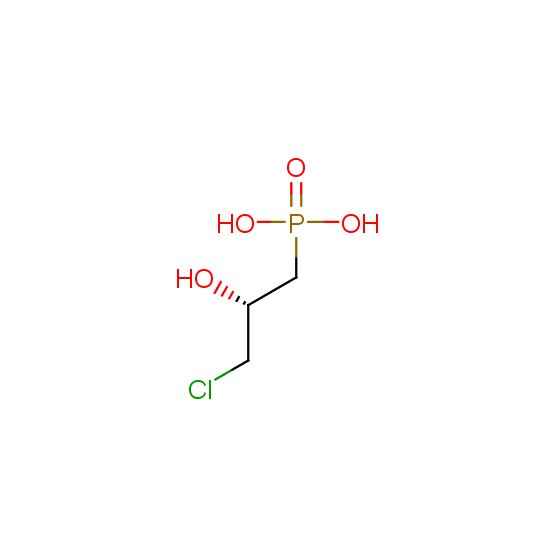
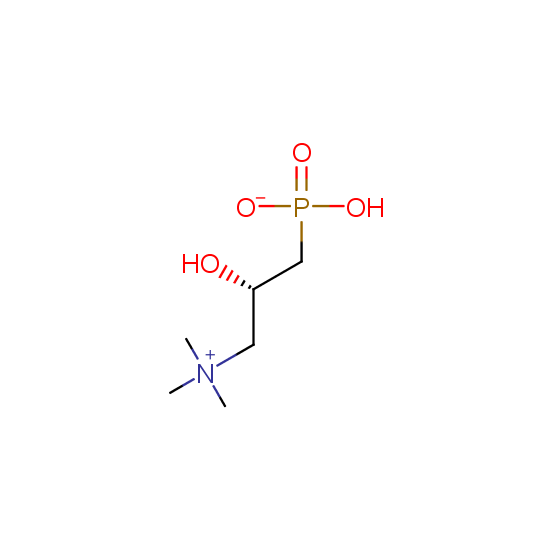
Hydroxyphosphonates and hydroxyaminophosphonates (source of chirality – catalytic asymmetric synthesis)