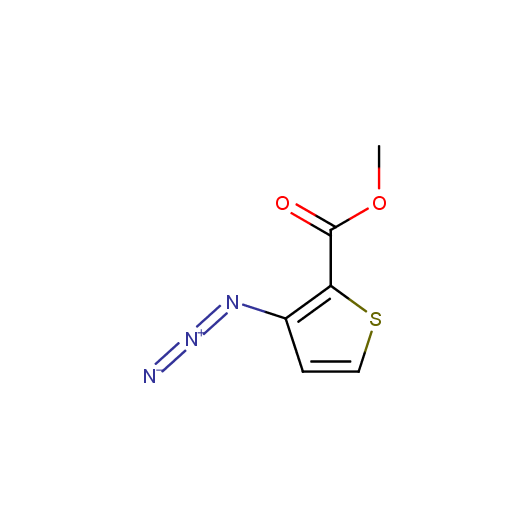
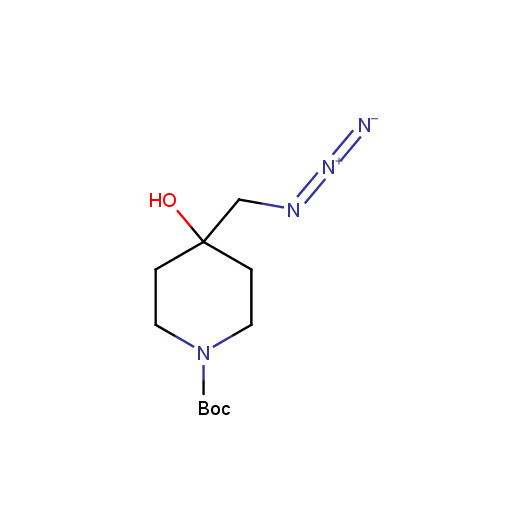
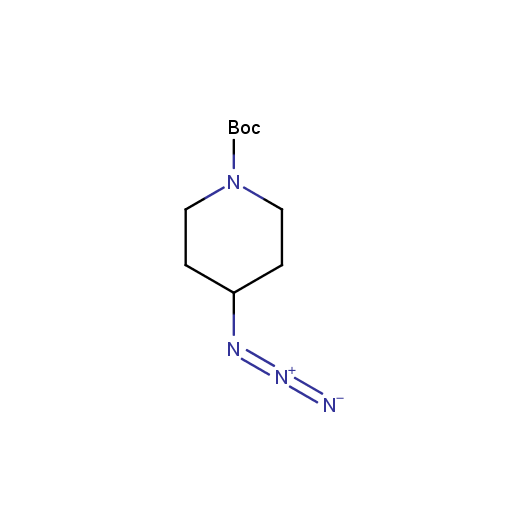
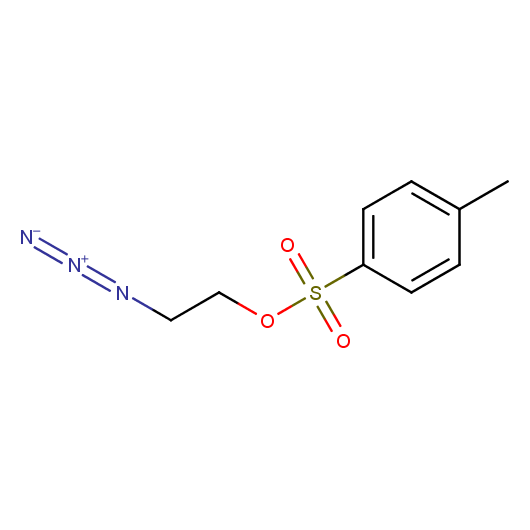
Organic azides are an important class of synthetic building blocks. In organic chemistry azides are commonly used for introducing an amino-group via Staudinger reaction. With the recent advent of "click-chemistry" azides became enormously popular for their participation in the Cu(I)-catalyzed Huisgen azide-alkyne 1,3-dipolar cycloaddition reaction. Because of the high rate of synthetic success of both Huisgen cycloaddition and Staudinger reduction these reactions are often used in combinatorial and medicinal chemistry. Enamine presented the set of azides suitable for click-chemistry transformations earlier.
Building blocks capable of linking of low molecular weight compounds in chemically and stereochemically predefined manners are one of the major demands of both high throughput parallel synthesis and fragment-based lead discovery. In this connection azides bearing additional functional groups are especially attractive building blocks. For example, 2-tosyloxyethyl azide was successfully employed in the preparation of analogues of nucleosides and nucleotides, amino acids functionalized with metal ligands 1, biologicaly potent vicinal diamines, and bimodal MR-PET contrast agents. Different amino-functionalized azides were used for the "click-to-chelate" installation of metal chelates 2 into biomolecules for radiolabeling and in the synthesis of D3 selective dopamine receptors 3. Azides bearing other functional groups were employed in the preparation of potent inhibitors of NAD synthesis 4 and for in-situ selection of lead compounds by click-chemistry. Besides medicine-oriented syntheses functionalized azides were used as key building blocks for efficient synthesis of polyamines, asymmetric vicinal diamines, and [n]rotaxanes.
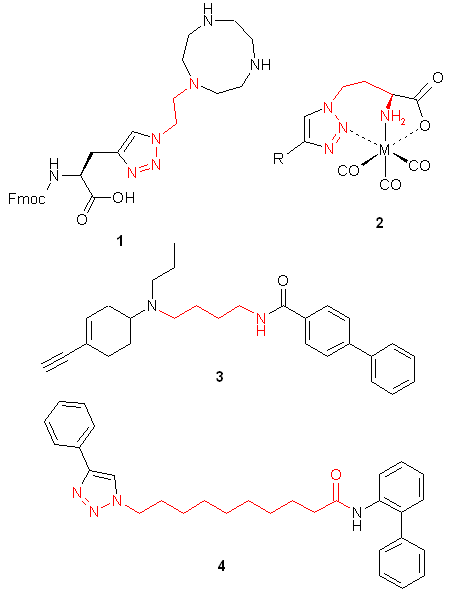
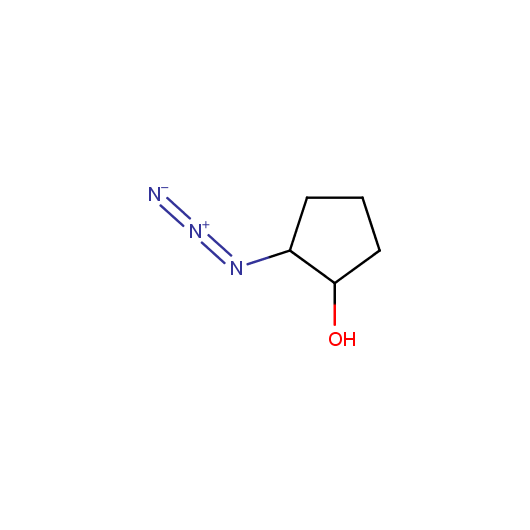
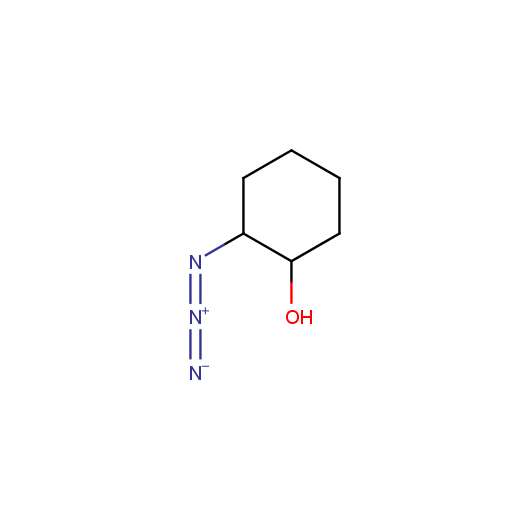
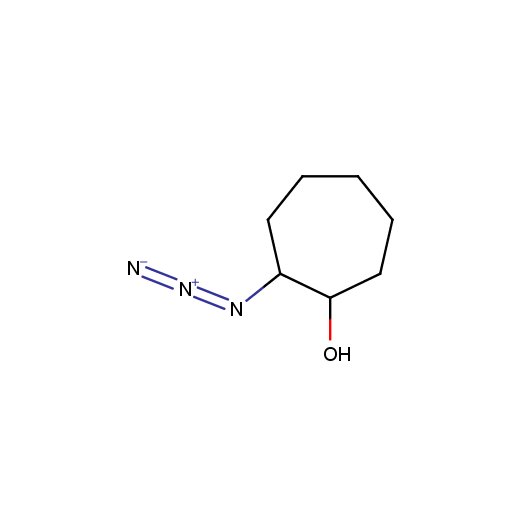
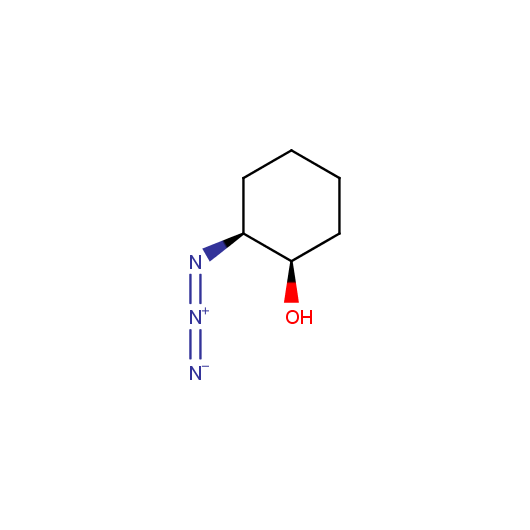
Given this synthetic potential we have generated a collection of functionalized azides. The collection contains both known and novel compounds. Examples of available functionalized azides are presented below.