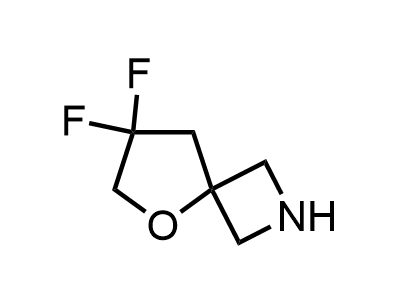
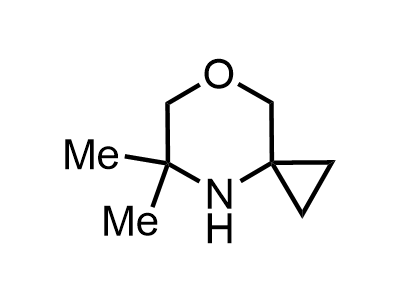
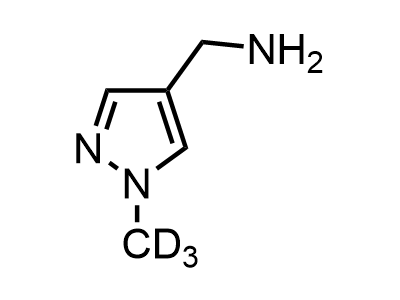
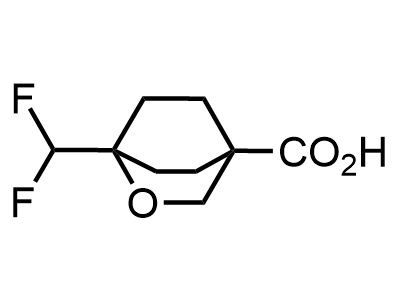
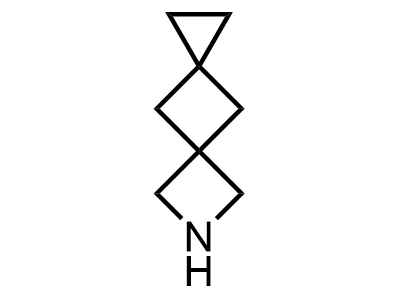
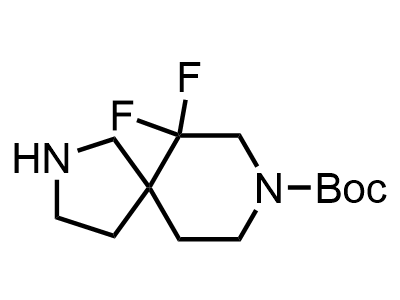
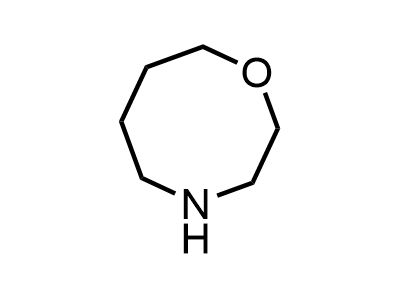
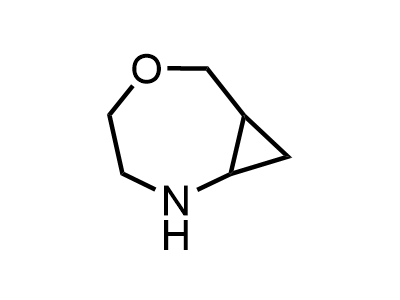
300 Thousand compounds in stock
Original and unique
Make-on-demand
Building Blocks
1B novel building blocks
Reliable supply
Over 650 highly skillful chemists
Unique synthesis technologies
48B Billion
REAL compounds and
Custom Library Synthesis
On site access to all Enamine stock BB’s
Highly flexible arrangements
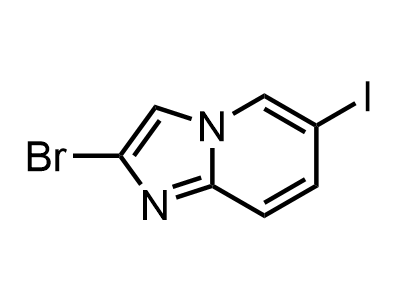
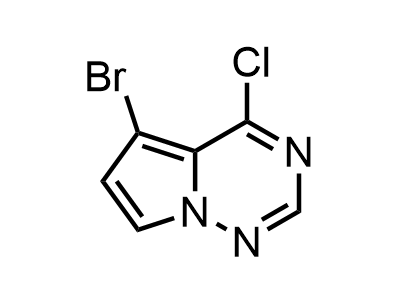
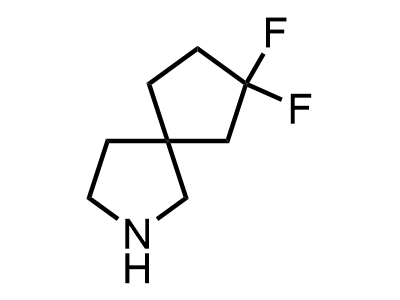
2 000 new building blocks are synthesized monthly. Here is an important update to our MedChem Highlights from March 2024
Recent News
07 May 2024
Press Release
Barcelona, Spain, and Kyiv, Ukraine, 7 May 2024. Pharmacelera, the leading provider of computational tools for hit discovery, and Enamine, the developer of the world’s largest and most reputable virtual space: REAL, have announced the extension of their current partnership to explore an extraordinary magnitude of compounds, that has been extended by a 10 fold factor – when compared to the early version. Ultra-large chemical libraries constitute a key paradigm to tap into new and still unexplored chemical spaces, increasing the probability for the researcher to find new and chemically diverse potent hits for Discovery Programs. Efficient handling of the ultra-large compound libraries still remains the main challenge.
29 April 2024
Press Release
Kemptthal, Switzerland and Kyiv, Ukraine, April 29, 2024: Synple Chem, an innovative developer of integrated automated chemical synthesis solutions, announced today a strategic partnership with Enamine, a world-renowned supplier of building blocks. The partners agreed to jointly develop a new chemical space by fueling the world’s largest collection of building blocks provided by Enamine to Synple Chem’s reaction outcome prediction tools. Researchers will be able to download the resulting compound library and search it using their workflows. Selected compounds will be synthesized by Synple Chem’s automated synthesis platform.
11 April 2024
Press Release
Cambridge, UK and Kyiv, Ukraine, 11 April 2024: Metrion Biosciences Limited (“Metrion”), the specialist ion channel and cardiac safety screening contract research organisation (CRO) and drug discovery company, and Enamine Ltd (“Enamine”), the global leader in supplying small molecules and early drug discovery services, announced that Metrion has enhanced its High Throughput Screening (HTS) services with the addition of access to Enamine’s compound libraries.
Phys. Chem. Chem. Phys. , 2013, 15 (23), 8962-8971
DOI: 10.1039/C3CP50896J
Wadhwani P.; Reichert J.; Strandberg E.; Burck J.; Misiewicz J.; Afonin S.; Heidenreich N.; Fanghanel S.; Mykhailiuk P. K.; Komarov I. V.; Ulrich A. S.
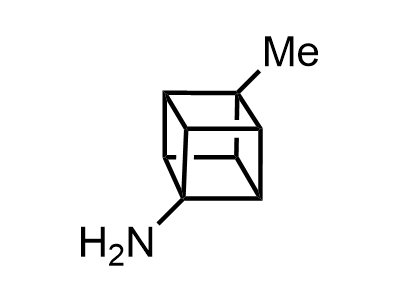
Single d-amino acid substitutions can be used to suppress or slow down the aggregation of peptides into β-sheeted assemblies compared to the respective L-amino acids. Here, we investigate the influence of local stereochemistry in the model peptide [KIGAKI]3-NH2, which is known to form amyloid-like fibrils. To find out whether aggregation plays a role in various biologically relevant functions that involve peptide-lipid interactions, we studied the antimicrobial, hemolytic and fusogenic activities of this amphiphilic membrane-active molecule. The stiff and sterically constrained amino acid CF3-Bpg [3-(trifluoromethyl)-bicyclopent-[1,1,1]-1-ylglycine] was incorporated either as an L- or a D-enantiomer at different hydrophobic positions of the KIGAKI sequence. D-Epimers have a higher aggregation threshold than the L-epimers, yet the aggregation of both was confirmed using electron microscopy and circular dichroism. Solid-state 19F-NMR analysis showed that the peptide aggregated in native membranes from human erythrocytes and bacterial protoplasts in the same way as in synthetic lipid bilayers. We then monitored the effect of the single L- or D-CF3-Bpg substitutions in KIGAKI on its distinct biological activities, which have to be measured at low peptide concentrations where the aggregation threshold cannot be directly assessed. These functional assays showed that the aggregation propensity of KIGAKI does not play a role in its antimicrobial action, but an increased tendency to aggregate promotes other undesirable effects such as hemolysis and membrane fusion. These results confirm the membranolytic and thereby toxic nature of amyloidogenic peptides, and emphasize the unpredictable role of peptide aggregation in the different assays used to study biological activities.
![Stereochemical effects on the aggregation and biological properties of the fibril-forming peptide [KIGAKI]3 in membranes](/images/publications/C3CP50896J.png)
Wadhwani P.; Reichert J.; Strandberg E.; Burck J.; Misiewicz J.; Afonin S.; Heidenreich N.; Fanghanel S.; Mykhailiuk P. K.; Komarov I. V.; Ulrich A. S.
Phys. Chem. Chem. Phys. 2013, 15 (23), 8962-8971
DOI: 10.1039/C3CP50896J
![Stereochemical effects on the aggregation and biological properties of the fibril-forming peptide [KIGAKI]3 in membranes](/images/publications/C3CP50896J.png)