Org. Biomol. Chem. 2015, 13 (11), 3171-3181
DOI: 10.1039/C5OB00034C
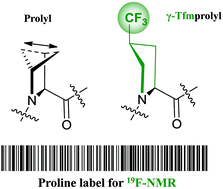
γ-(4S)-Trifluoromethyl proline was synthesised according to a modified literature protocol with improved yield on a multigram scale. Conformational properties of the amide bond formed by the amino acid were characterised using N-acetyl methyl ester model. The amide populations (s-trans vs. s-cis) and thermodynamic parameters of the isomerization were found to be similar to the corresponding values for intact proline. Therefore, the γ-trifluoromethyl proline was suggested as a structurally low-disturbing proline substitution in peptides for their structural studies by 19F-NMR. Indeed, the exchange of native proline for γ-trifluoromethyl proline in the peptide antibiotic gramicidin S was shown to preserve the overall amphipathic peptide structure. The utility of the amino acid as a selective 19F-NMR label was demonstrated by observing the re-alignment of the labelled gramicidin S in oriented lipid bilayers.