J. Org. Chem. 2015, 80 (24), 12258-11264
DOI: 10.1021/acs.joc.5b02171
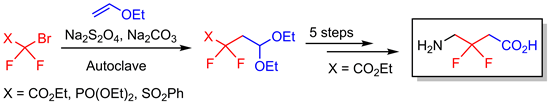
Addition reactions of perfluoroalkyl radicals to ordinary or polyfluorinated alkenes have been frequently used to synthesize perfluoroalkylated organic compounds. Here ethyl/methyl 2-bromo-2,2-difluoroacetate, diethyl (bromodifluoromethyl)phosphonate, [(bromodifluoromethyl)sulfonyl]benzene, and ethyl 2-bromo-2-fluoroacetate were involved in Na2S2O4-mediated radical additions to vinyl ethers in the presence of alcohols to give difluoro or monofluoroacetyl-substituted acetals or corresponding difluoromethylphosphonate- and (difluoromethylphenyl)sulfonyl-substituted alkyl acetals. This methodology has also been applied as a key step in the synthesis of hitherto unknown 3,3-difluoro-GABA, completing the series of isomeric difluoro GABAs. Comparison of the pKa values of 3-fluoro- and 3,3-difluoro-GABA with that of the fluorine free parent compound showed that introduction of each fluorine lead to acidification of both the amino and the carboxyl functions by approximately one unit.