Substituted ureas are drawing considerable attention as pharmacologically relevant compounds. Our proprietary trifluorourethanes, reported earlier, are suitable precursors for the preparation of aryl/heteroaryl-alkyl or aryl/heteroaryl-dialkyl urea derivatives via parallel synthesis, but they are not appropriate for the preparation of ureas bearing different alkyl substituents. We report now a new set of building blocks, carbamoylimidazoles, specifically designed for the synthesis of ureas with multiple alkyl groups.
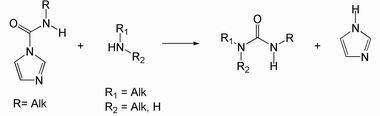
Carbamoylimidazoles derived from primary amines react with primary and secondary aliphatic amines under mild conditions to yield the corresponding urea derivatives:
Carbamoylimidazoles derived from secondary amines are less reactive due to steric hindrance. However, when converted to tertiary salts in situ, they are more labile, and react with primary and secondary aliphatic amines readily:
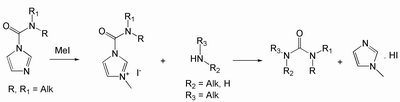
Isolation of alkyl-substituted ureas with low water solubility is accomplished by removal of side products, imidazole or methylimidazole iodide, with an aqueous work up followed by re-crystallization of the desired products from appropriate organic solvents. Water-soluble urea derivatives can be purified by acidic ion-exchange resins. When basic functional groups are present in the product or purification from alkylimidazole salt is required, then the final product can be purified by HPLC or other chromatographic methods.
Carbamoylimidazoles are stable, have extended shelf life, and are available in stock. Chemists of Enamine have developed efficient procedures for the preparation of carbamoylimidazoles from different amines, accordingly, synthesis of custom building blocks can be achieved. Furthermore, we have used the carbamoylimidazoles and trifluorouretanes building blocks to add urea derivatives to our Screening Collection.
