This issue of Enamine Product Focus represents a family of Building Blocks containing Hydrazine unit. Several aspects of utilizing the hydrazine building blocks for the design of potential drug candidates can be outlined. Despite hydrazines themselves are rarely thought as promising drug candidates, some successful examples of drugs possessing hydrazine moiety can be found, e. g. antiparkinsonic agent Carbidopa (Lodosyn®), antidepressant Phenelzine (Nardil®) or vasodilator Hydralazine (Apresoline®).
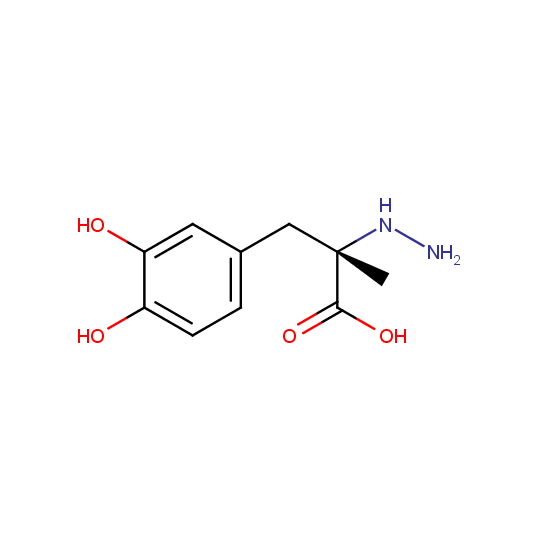
Carbidopa
Phenelzine
Hydralazine
An interesting application of hydrazines in the design of potential drug candidates evolved with the idea of amino acids and peptides modification. If the Ca atom of an amino acid residue in a peptide is replaced by Nitrogen, an aza peptide is obtained.
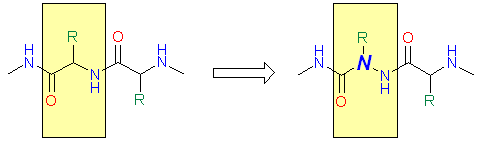 |
|
| Peptide | Aza peptide |
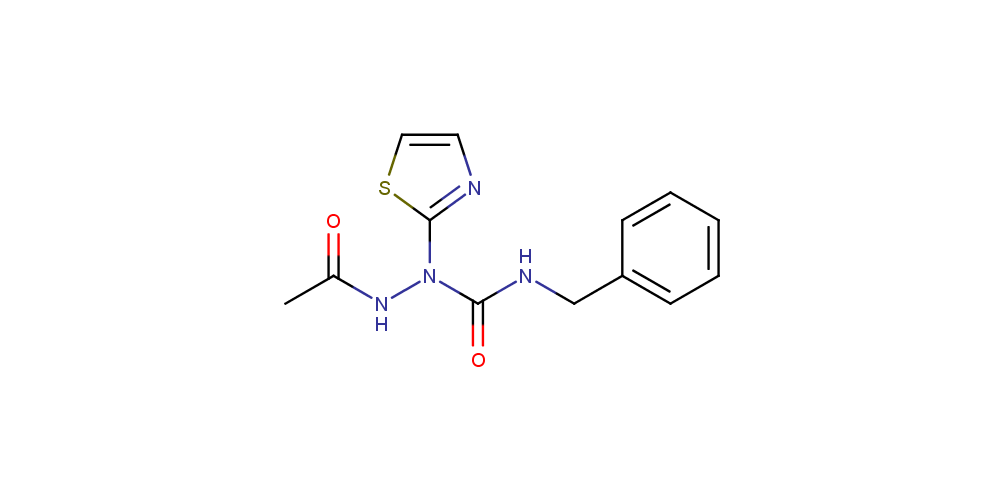
The potential of peptide therapeutics has gained increased attention recently due to the potency, specificity and low toxicity of peptide drugs. Modified peptides (in particular, aza peptides) are also attracting growing interest as they exhibit higher biodegradation stability comparing to parent peptides. Some of aza peptide drug candidates that reached preclinical phase are shown below.
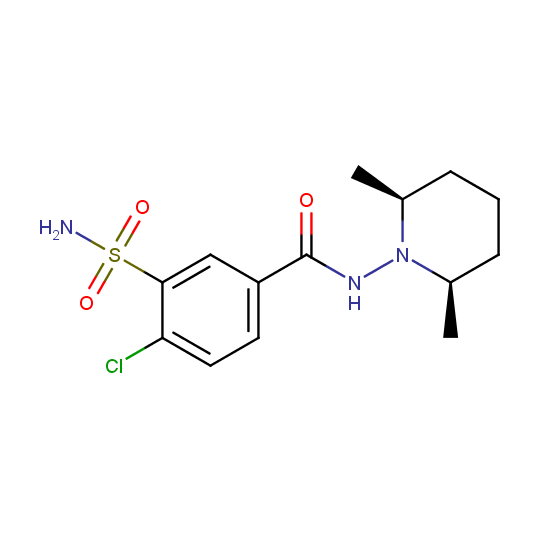
Other acyl derivatives of hydrazines are also found among drugs or drug candidates, e. g. diuretic Clopamide (Brinaldrix®) and antidepressant Isocarboxazid (Marplan®).
Celecoxib
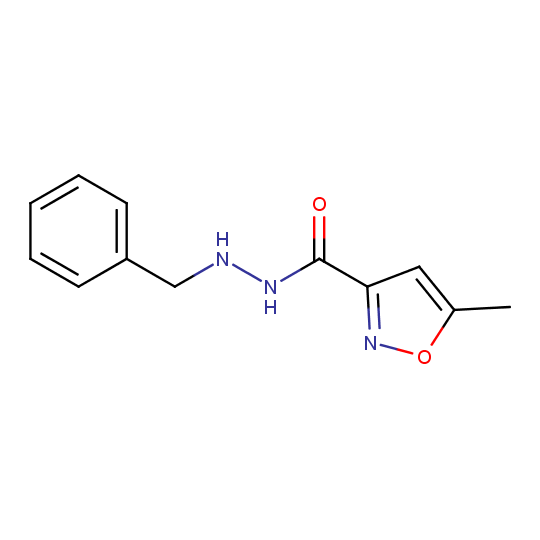
Isocarboxazid
Another use of hydrazines in drug design is related to the concept of “Click Chemistry” introduced by H. C. Kolb, M. G. Finn and K. B. Shaprless. The main idea behind the “Click Chemistry” is the use of mild, highly reliable, selective and powerful reactions for the rapid synthesis of large arrays of chemical compounds. One of the reactions identified to be suitable for Click Chemistry is condensation of Hydrazines with carbonyl compounds. The most important particular case of these reactions is heterocyclic compounds (e.g. Pyrazoles, Piridazines and 1,2,4-Triazoles) formation from dicarbonyl compounds. Many examples of Pyrazole-containing drugs can be found including anti-inflammatory drug Celecoxib (Celebrex® or Onsenal®) and Regadenosin (Lexiscan®), which is used as stress agent in radionuclide myocardial perfusion imaging.
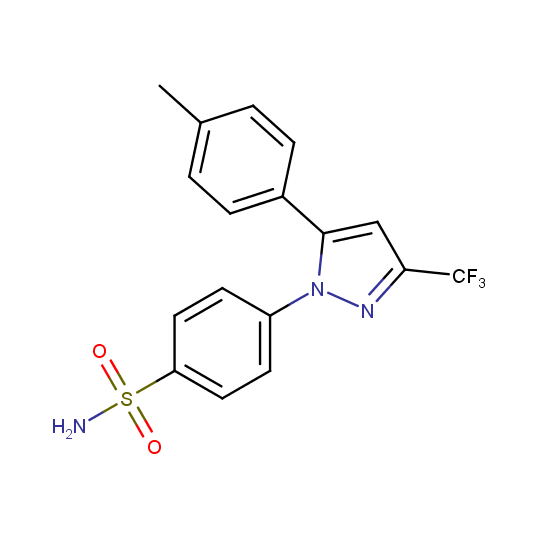
Celecoxib
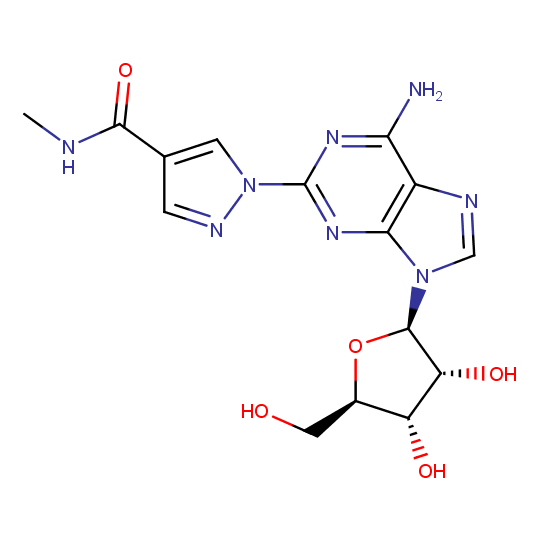
Regadenosin
Enamine offers several diverse subsets of Hydrazine building blocks including monosubsituted (both aliphatic and aromatic) and disubstituted hydrazines, cyclic hydrazines, acyl and sulfonyl hydrazines. Procedures for the synthesis of hydrazines were elaborated, and their scope and limitations were established. The methods depicted in the scheme below allow obtaining a large diversity of hydrazine building blocks at 1–10 g scale; novel compounds of the requested structure can be obtained in 4–6 weeks. Scale-up to 1 kg can be performed upon request. Moreover, protected derivatives at any of the Nitrogen atoms of the hydrazine moiety can be obtained. Applying the Mitsunobu conditions to chiral alcohols as starting materials gives a possibility for the synthesis of enantiopure chiral alkyl hydrazines. Further in-house modification of hydrazine building blocks is also possible.
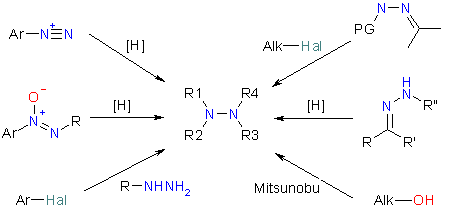
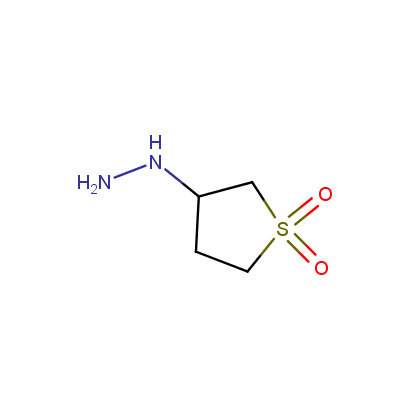
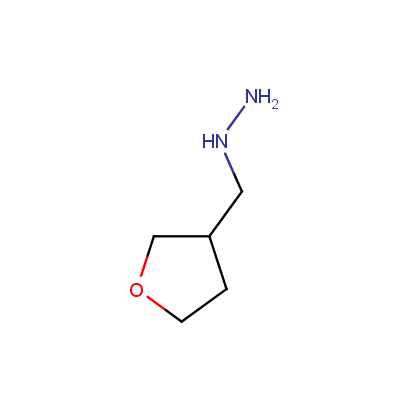
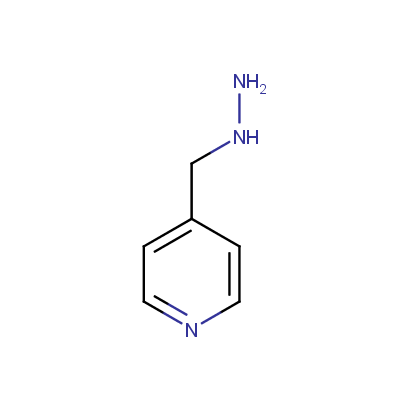
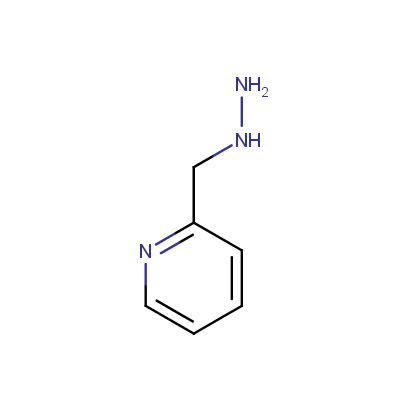
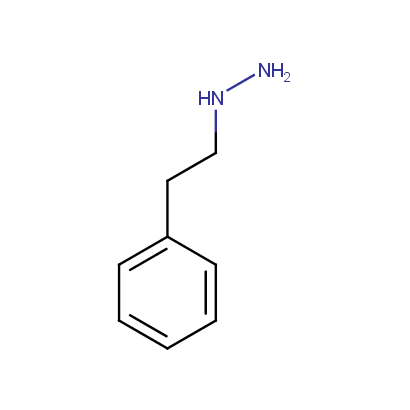
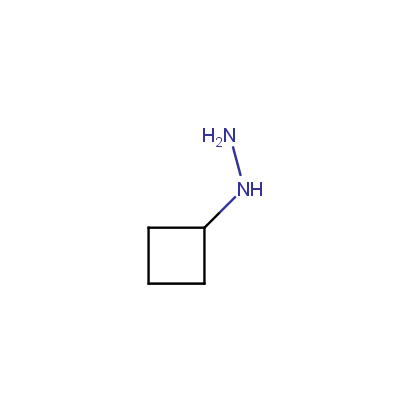
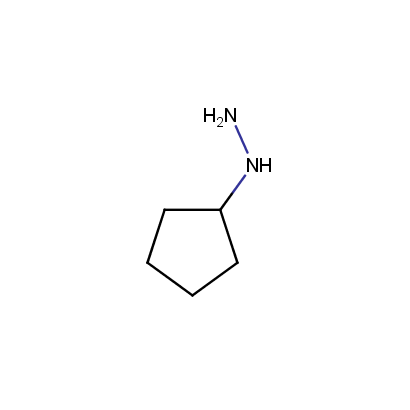
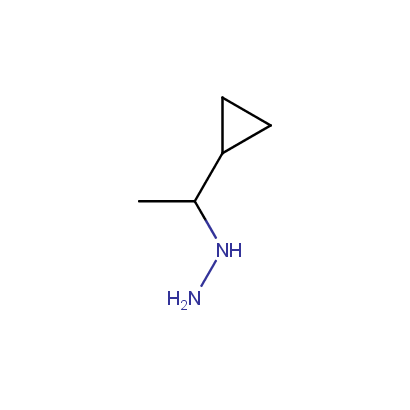
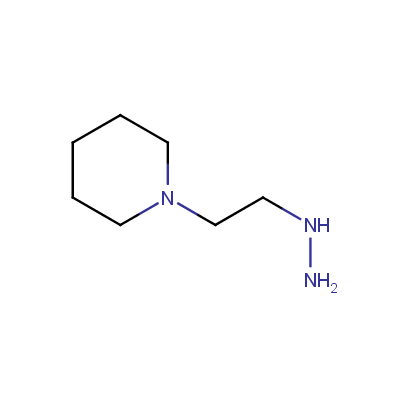
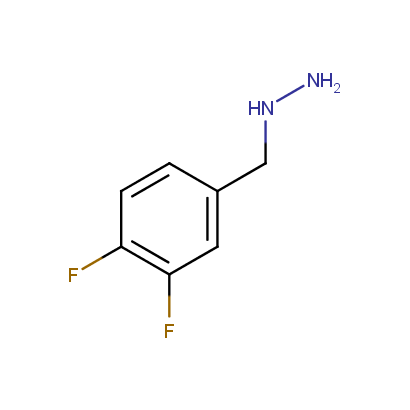
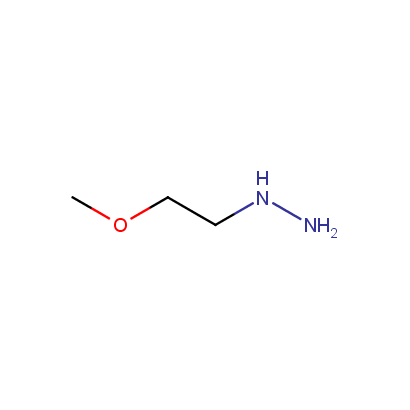
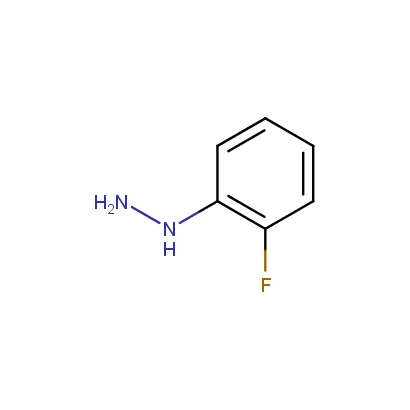
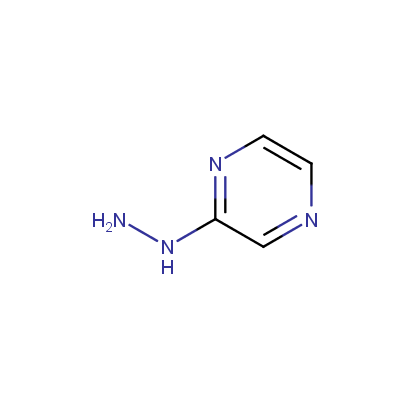
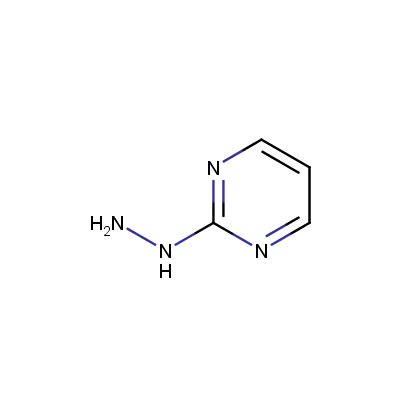
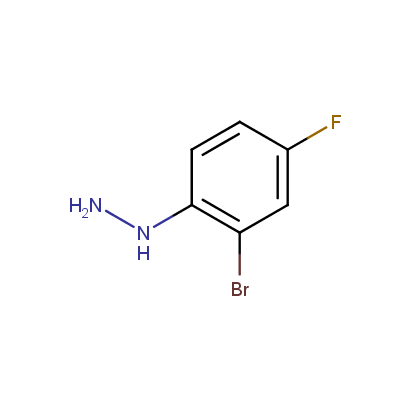
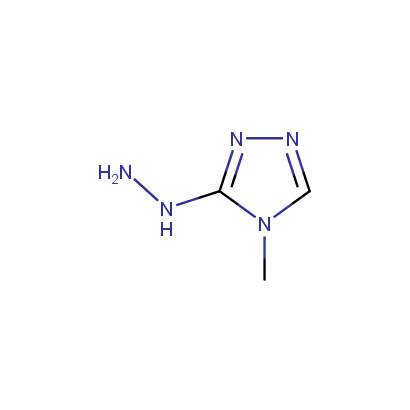
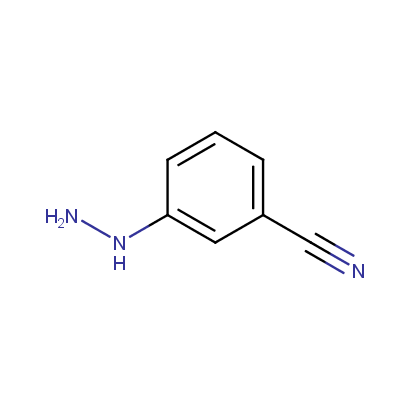
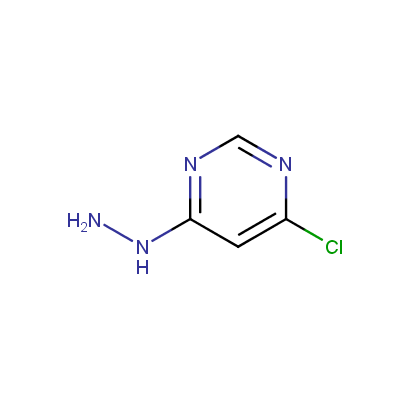
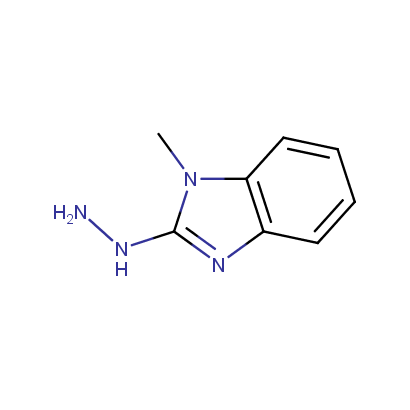
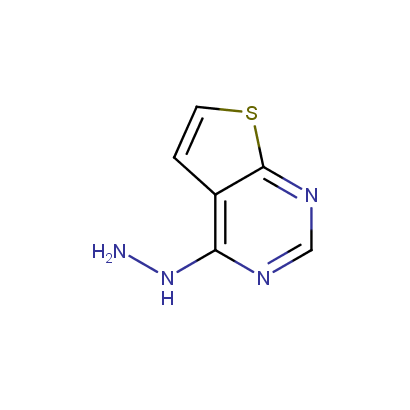
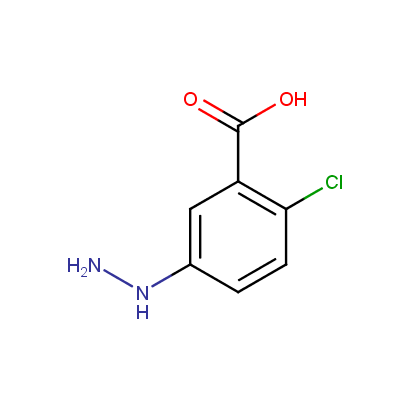
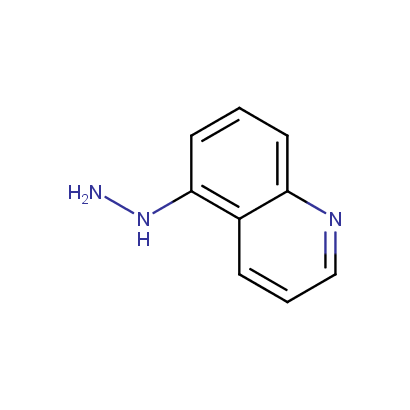
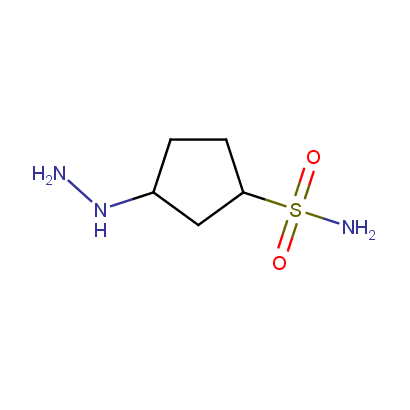
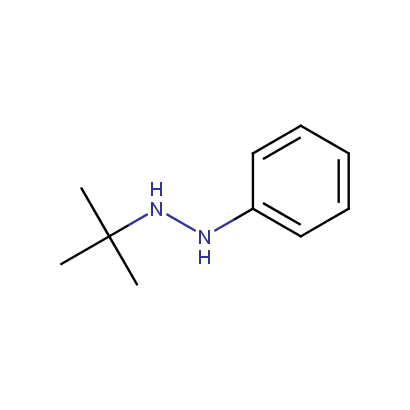
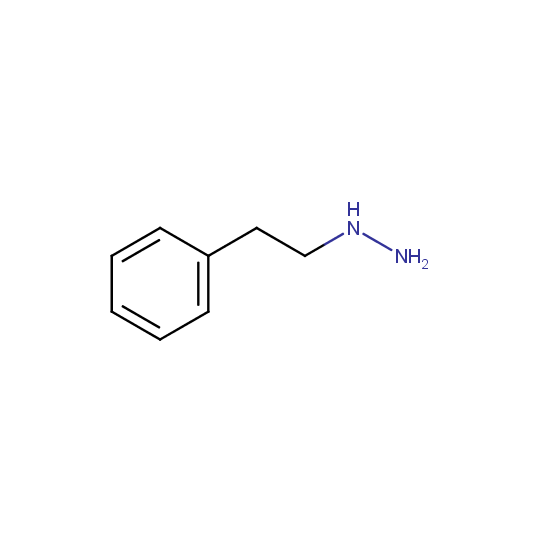
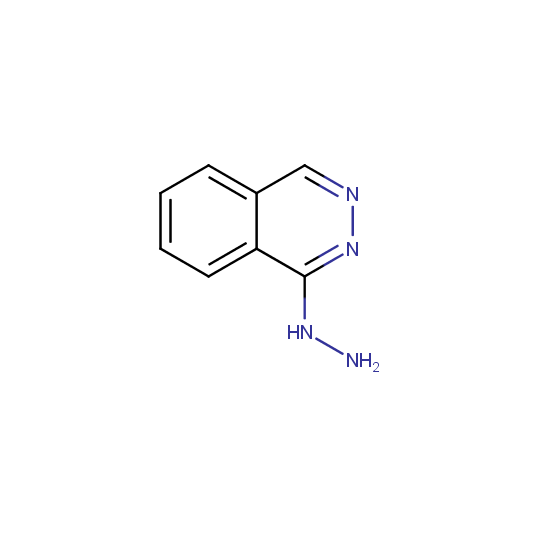
Some examples of the hydrazines are shown below.
Monosubstituted Aliphatic Hydrazines
Monosubstituted Aromatic Hydrazines
Disubstituted Hydrazines
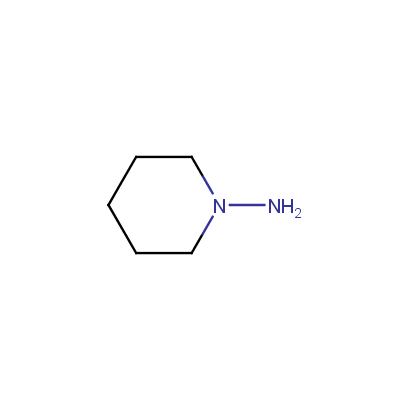
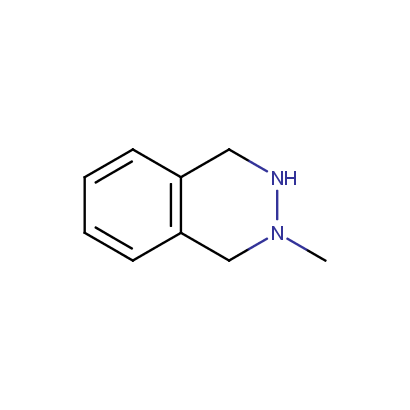
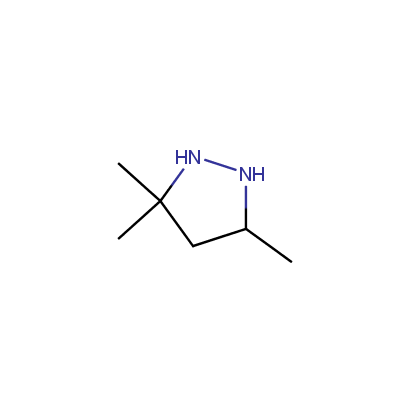
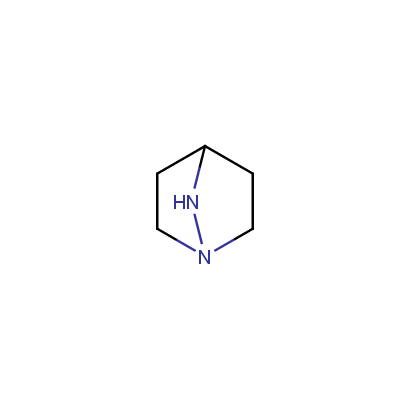
Cyclic Hydrazines
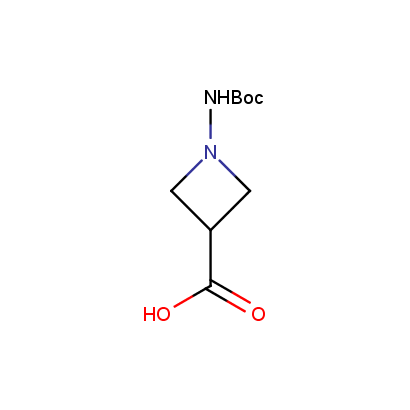
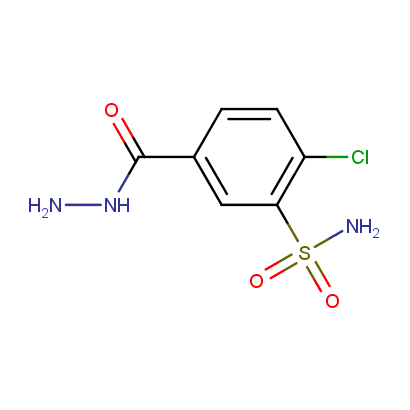
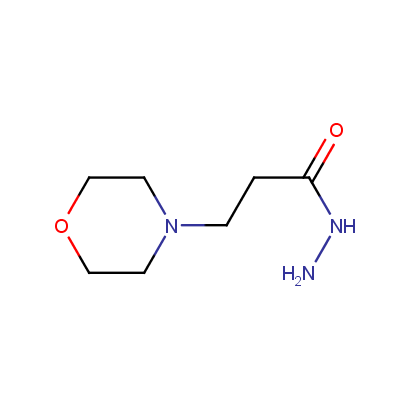
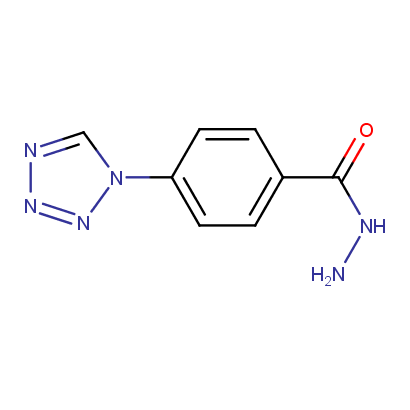
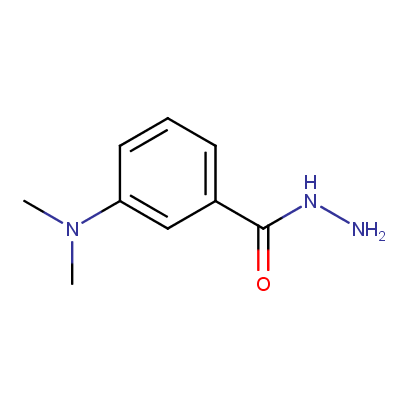
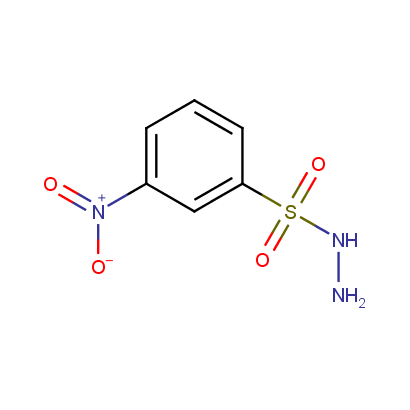
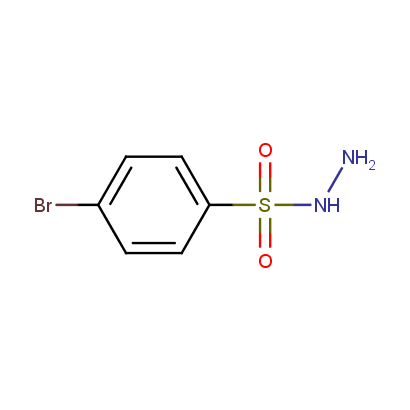
Acyl and Sulfonyl Hydrazines