We offer cost-effective chemistry support to any medchem-related projects including synthesis of sophisticated individual compound and unchallenged parallel synthesis of small focused libraries besides conventional wet chemistry. Enamine is a non-classic CRO, rather a chemistry research organization. Having the largest proportion of its business in compound catalog sales, the company has accumulated over the years at its research site in Kyiv the world’s largest collection of building blocks. You can access this powerful resource directly at Enamine when outsourcing your chemical FTE’s to us. This will allow you to:
- Decrease turnaround time by having same day supply to your FTE labs
- Access unique compounds available exclusively from Enamine
- Save on costs
Our proven high potential to accelerate and enhance drug discovery projects has been recognized by a recently drastically growing number of clients – from academic labs to big pharma companies via SME’s. The number of chemical FTE’s outsourced to Enamine has doubled since 2019. Currently only 1/3 of our chemists are engaged in FTE collaborations and we can easily expand our service branch by relocating additional chemists from non-exclusive synthesis for our public catalogs.

Skillful chemists
Enamine is a global leader in synthetic innovation in medicinal chemistry. We have over 650 skillful chemists with scientific expertise in various fields of chemistry widely documented in the peer-reviewed scientific journals. Our chemists have in-depth experience in a wide number of unique chemical techniques such as fluorination with SF4, photochemistry in flow, electrochemistry, high-pressure hydrogenation etc., which are well validated at Enamine and are scarcely available at other CRO’s.
Building Blocks
Our FTE rate includes free-of-charge access to most of our building blocks and the rest is offered with a sizeable discount. Our clients benefit from our enormous experience in building block synthesis. Enamine’s collection of make-on-demand “MADE” building blocks provides an important source of ideas and inspiration.
Parallel synthesis capabilities
We can use parallel synthesis for more than 35 different reactions including photochemistry. Based on the deep knowledge of our building blocks and on their reactivity we have developed predictive models which can maximize success rate in synthesis of compound libraries. Read more…
Molecular modeling
If needed Enamine Molecular modeling group can support the project with virtual screening, pharmacophore modeling and other CADD services.
Flexible arrangement
In contrast to classical CRO’s, the number of FTE’s at Enamine can be amended on a ultra-short notice, should it be to increase or decrease the size of a team.
ADMET/PK tests and HTS
Bienta, the biological department of Enamine (www.bienta.net), can deploy state-of-the-art support to medchem collaborations by providing ADMET and Pharmacokinetics tests including animal studies. If a new hit-finding campaign is required, Bienta can offer HTS using Enamine’s significant diverse screening libraries or any customizable set from targeted and diverse libraries pools available at Enamine, which are totaling over 4.6M compounds. Read more…
All these features and more make Enamine a partner of first choice for different types of FTE-projects including:
- Synthesis of hit-finding libraries, analog preparation;
- Scaffold decoration using diverse capping agents;
- Singleton synthesis, Hit-to-lead optimization, scaffold-hopping;
- Synthesis of Building-Blocks / scaffolds including scale-up validation;
- Feasibility studies.
In drug development, great attention is paid to the availability of new pharmaceutical products and their quality. The crucial stages of the drug development process are the identification, quantitation, and control of pharmaceutical impurities. The quantity of unwanted compounds determines the overall safety of the final pharmaceutical product. The restrictions of the impurity content in active pharmaceutical ingredients (APIs) and drug formulations are given in such compendia as USP, EP, BP, JP, and ChP.
Enamine has precisely documented scientific expertise in organic synthesis and analytical chemistry, which empowers the synthesis and identification of previously unidentified impurities. Our catalog includes 692 drug impurity reference standards in the stock. The certificates of analysis are given for all compounds, they include clear-cut identity and purity data validated using NMR, HPLC/MS, and/or GC/MS methods.
Download
692 compounds
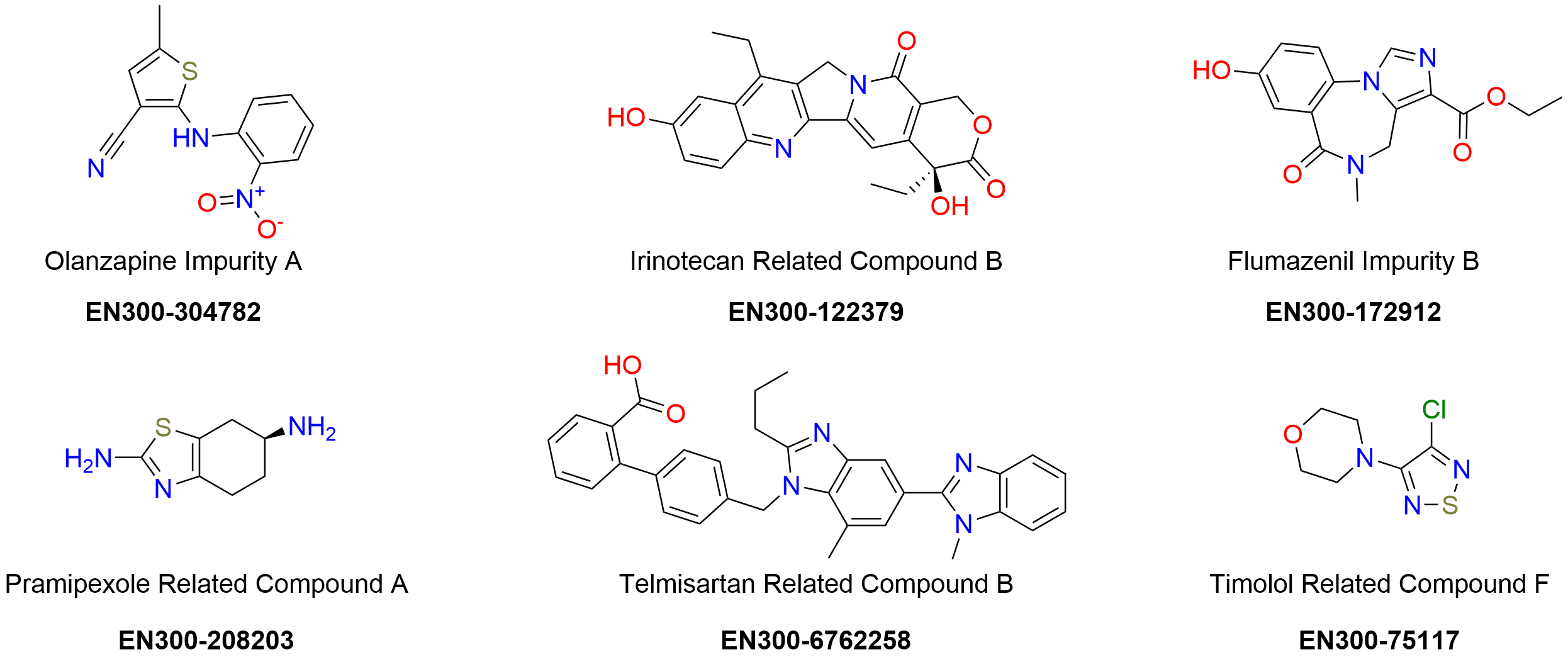
Drug impurity profiling
Enamine provides services in the analysis of API’s to identify impurities. We investigate all steps in the production process assessing any compound or solvent involved in it that could possibly be responsible for the formation of impurities or degradation products. This comprehensive analysis allows a reliable prediction of the structure of the unknown impurities, identification of their generation mechanism, and subsequently strategic impurity management.
Custom Synthesis
Our strong point and competitive advantage are in the design of synthesis routes. We don’t require documented synthesis procedure to produce your compound of interest. In most of the cases, we can propose a realistic synthesis scheme from scratch and successfully realize it producing a desirable amount of compound (from mg to gram scale) in the requested purity.
Pharmacokinetics
Pharmacokinetics (PK) reveals the fate of substances in the body over time. The compounds can be administered in multiple ways, among which peroral (PO) and intravenous (IV) are the most popular ones. Cassette dosing in the PK study allows for understanding of the drugs interaction in vivo system.
All PK studies can be customized upon request.
In vivo models
In vivo models play a pivotal role in the drug discovery process. Evaluation of the lead compounds in living organisms for safety and efficacy remains one of the main requirements before clinical studies in humans. In our studies, we thrive to minimize the use of vertebral animals and substitute assays for evaluation of the lead compounds with in vitro models whenever possible. However, some complex diseases, e.g. diabetes, require enrolment of experimental animals.
Our bioanalytical laboratory possesses the national regulatory certification for providing bioanalytical support of clinical bioavailability and bioequivalence studies.
We work closely with our customers to develop an ADME/PK program specifically tailored to their project needs, ensuring flexible approach depending on the requirements and specifications. We can develop or modify specific methods according to what is requested, including high-throughput ADME analysis and Express ADME panel to accomodate large sets and strict deadlines.
Animal Studies
Rodent Models
High-Throughput Screening
Having the world's largest screening collection, we established our proprietary High-Throughput Screening Center to enable in-house support for hit finding projects. You can select from a suite of pre-plated diversity or targeted libraries and our fragment collection, or simply cherry-pick the compounds from 4.6M+ compounds stock for immediate screening next door.
- Assay development and validation
- Biophysical, biochemical, and cell-based assays
- All common assay optical readouts
- Adaptable to majority known targets
- Recombinant protein expression/purification services
- Direct access to the world's largest compound library
- Hit confirmation only from fresh powders
- The lowest possible cost access to Enamine compounds
Our services
We prepare assay-ready plates for your screening campaigns using Labcyte Echo 550 acoustic dispenser at any format (96-, 384- or 1536-wells) and any set size.
Screening readouts:
- UV-VIS absorbance
- luminescence
- fluorescence intensity
- fluorescence polarization
- time-resolved fluorescence/energy transfer (FRET, TR-FRET, HTRF, DELFIA, LANCE)
- Surface Plasmon Resonance (SPR)
- cellular calcium mobilization (FLIPR)
- Thermal Shift Assay (TSA)
- ELISA
- AlphaScreen
- enzyme/reporter assays
- cell viability assays
Our Smart Screening Technology is based on the extensive use of our chemoinformatics and molecular modeling resources and iterative access to the on-site compound repository of over 4.6M compounds after each round of experimental screening of in-silico selected compound sets. Transition to screening of new analogues which are not available in stock for hit expansion and SAR building is profoundly facilitated with REAL compound technology. Enamine can assemble billions of new compounds in just 1 protocolled synthesis step. Speed of supply and success rate attained are so high that the compounds are sourced as if from stock. In this way the Smart Screening Technology enables the rapid expansion from screening of relatively small diverse screening library to focused exploration of billions of new compounds. Synthetic chemistry support of hit and lead optimization, as well as optional in-house ADMET/PK testing and animal efficacy studies provide a comprehensive follow-up capability.
Screening facilities
Our Screening Center is equipped with 7 robotic liquid handlers (Thermo, Tecan, Beckman-Coulter, CyBio), 11 multifunctional plate readers (Molecular Devices, Tecan, BMG), including the state-of-the-art SpectraMax® Paradigm® (Molecular Devices) and PHERAstar® FSX (BMG) readers, as well as various accessory equipment (multichannel dispensers, stackers, shakers, incubators, etc.).
– FLIPR® (Fluorometric Imaging Plate Reader) Tetra High Throughput Cellular Screening System (Molecular Devices) is a screening tool of choice for GPCRs and certain ion channels. The system can be used with both fluorescent and luminescent assays;
-Protein Thermal Shift Screening Platform (or Differential Scanning Fluorimetry) includes three ViiA 7 Systems (Applied Biosystems).
-Surface Plasmon Resonance (SPR) by OpenSPR XT (Nicoya Life Sciences) for studies of target-binding parameters.
ADME
With our in vitro ADME services we provide accurate and valuable information so important in early stages of drug discovery and development. Being in close proximity to the huge collection of ready-to-use screening compound libraries we can reduce the report delivery time or easily improve and facilitate the selection of the lead compounds.
