L-arginine (or Secretagogue (Caerulein)) acute pancreatitis models in rats.
Background:
L-arginine is widely used in therapy as a substrate for neuronal, inducible, and endothelial nitric oxide synthase. The L-arginine dose may vary depending on the required severity of pancreatitis (amount of acinar cells that died). Secretagogue increases protein-rich secretion of the pancreas (hyperstimulation). This leads to a 75% reduction of the enzyme stores in the exocrine part of the pancreas and results in pancreatic injury.
The model progress is characterized by acute pancreatic inflammation, edema infiltration of leukocytes, capillary dilatation, and microfocal parenchyma necrosis.
Service details:
A standard study design includes 3 groups of Wistar rats (vehicle dosed, treated with reference compound, and tested compound treated group), 5 rats per group, 7 days of acclimatization period, single or multiple L-arginine injections, 7 days of the treatment period. Animals are monitored for mortality, body weight changes, and signs of toxicity daily for the duration of treatment and the post-treatment period. Rats’ treatment procedures include invasive (by IV, IP, SC) and non-invasive (PO and diet incorporated) routes of drug administration. Clinical chemistry parameters of pancreas pathology development (level of amylase, trypsinogen, lipase in the blood) are determined over time (0, 2, 5, and 7 days after the administration of L-arginine). A gross necropsy is performed on all animals at the endpoint. Specific tests, such as hematological, urinary, and blood clinical chemistry analysis, food intake, histopathology, etc., are available on request.
The osteoporosis model in rats is achieved by ovariectomy procedure. Ovariectomy in adult animals induces estrogen deficiency and subsequent bone loss with corresponding clinical manifestations of postmenopausal osteoporosis.
Service details:
In a standard design, female rats (Sprague-Dawley or Wistar) are ovariectomized at age 6 months (or later – on-demand). The earliest signs of bone loss after ovariectomy are observed in 2 weeks after surgery. Therefore pathology-dependent treatment begins in 1-2 weeks after ovariectomy (OVX). Animals are divided at least into three groups: sham-operated control (SHAM), ovariectomized vehicle-treated control (OVX-V), and compound treated group (OVX-Cpd) (n=8 per group).
After X days of treatment, bone architecture and indices of bone fragility (trabecular thickness, number, separation, etc) are evaluated in proximal tibia by histomorphometry. Animals are observed for mortality and signs of toxicity daily for treatment and post-treatment period. Specific tests, such as urinary and blood clinical chemistry analysis, and bone turnover markers are available on request. These include bone formation markers: alkaline phosphatase, osteocalcin, and aminoterminal propeptides of procollagen type I (P1NP) in the serum. Bone resorption markers including carboxy-terminal cross-linking telopeptide of type I collagen (CTX), pyridinoline, and deoxypyridinoline (in both serum and urine). Inclusion of specialized endpoints based on the pharmacology of the test article is also available.
Tumor Necrosis Factor (TNF-α) is a proinflammatory cytokine with a significant ability to induce inflammation. Single parenteral administration of TNF-α is used as a model of sepsis and accompanying pathologies such as hypothermia, multiple organ injury including the lung, kidney, and other.
Service details: A standard study design includes at least 3 groups of male C57BL/6 mice (vehicle dosed, treated with reference compound, and tested compound treated group), 10 mice per group, 7 days of acclimatization period, a single injection of TNF-α, 3-4 days of the treatment period. Mice treatment procedures include intravenous TNF-α injection (50 μg endotoxin-free murine TNF-α alone or in combination with zVAD, body temperature was kept at 37°C to delay lethal shock), single or repeated dose drug administration. Mortality, body temperature, or customized clinical or physiological parameters are determined. Gross necropsy, specific tests, such as hematological, urinary, and blood clinical chemistry analysis, food intake, histopathology, etc. in combination with more definitive toxic or gross pathology endpoints are available on request.
Deliverable: A detailed study report including the description of the complete study design, full experimental data, and interpretation.
Animal models are essential for exploring acute renal failure (ARF) and the development of effective therapy for its optimal management. At Bienta we utilize several rodent models to mimic the clinical conditions of renal failure.
Cisplatin-induced acute kidney injury. Cisplatin is a widely used anticancer drug accompanied by adverse side effects, including toxin-induced acute kidney injury (AKI). Intraperitoneal administration of 10–12 mg/kg Cisplatin results in cellular uptake and efflux, apoptosis, vascular injury, oxidative and endoplasmic reticulum stress, and inflammation within 72 hours, followed by a slow recovery phase for 7 days. The severity of the pathology depends on the dose administered.
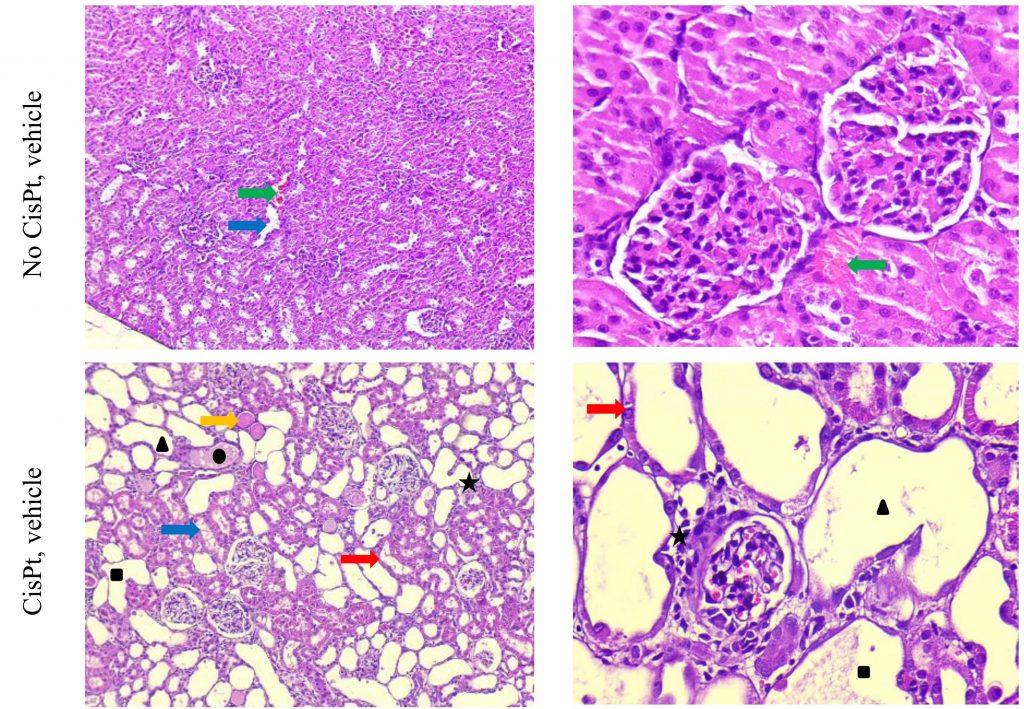
Lipopolysaccharide (LPS) induced acute kidney injury. LPS is a potent inducer of inflammation. Parenteral administration of LPS causes the production of a wide range of proinflammatory cytokines and is used as a model of sepsis. The inflammatory process also leads to hemodynamic changes and acute kidney injury. Intraperitoneal administration of 10 mg/kg LPS results in sepsis-associated acute renal damage within 24-72 hours. This is a rapid acute model, lasting 72-96 hours.
Gentamicin-induced acute kidney injury in rats Gentamicin is a broad-spectrum antibiotic with side effects of toxin-induced acute kidney injury. Intraperitoneal administration of 100 mg/kg Gentamicin for four consecutive days results in renal tubular damage in rats, followed by a slow recovery phase for 14 days. The severity of the pathology depends on the dose administered.
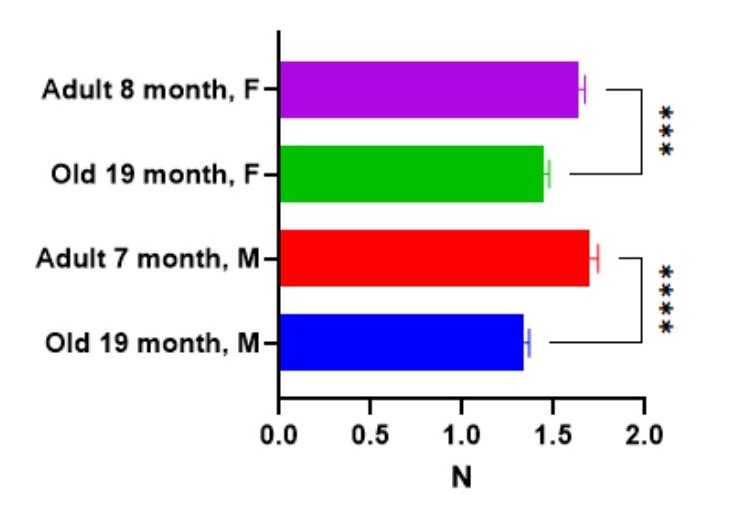
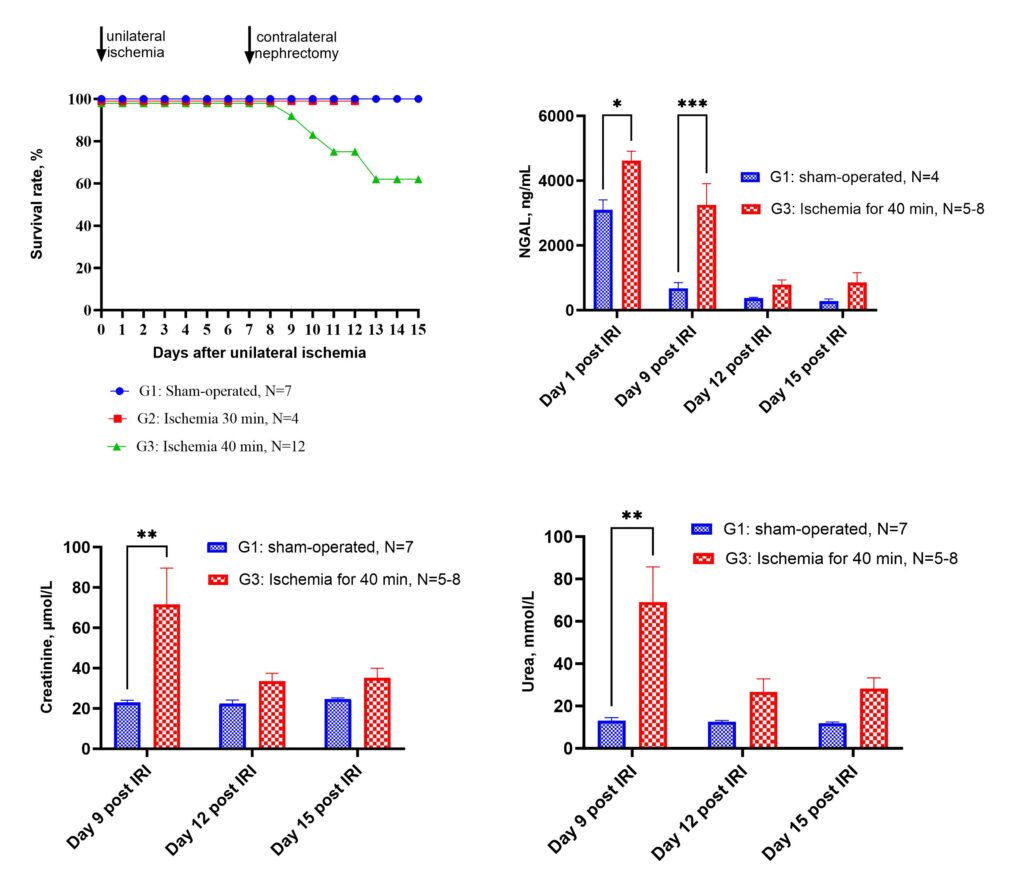
Renal ischemia-reperfusion injury model in rats The Renal ischemia-reperfusion injury model (IRI) is achieved by applying ischemia on the kidney by clamping the renal artery and vein. Typically, renal vascular clamping for 50 minutes is used to induce renal injury, followed by reperfusion. The ischemia-reperfusion procedure leads to an acute decrease in renal function, uncovered by the level of urea and creatinine in the blood.
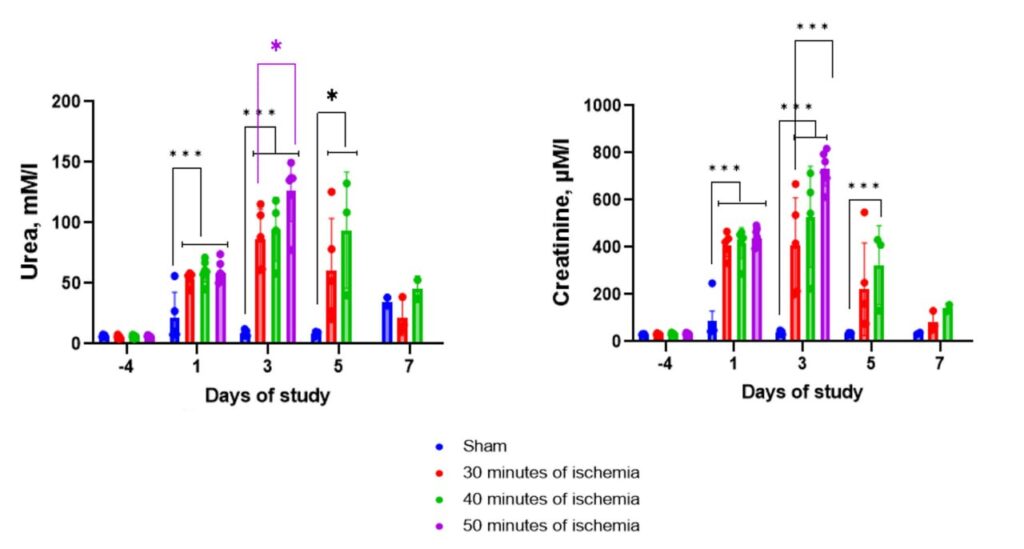
Renal unilateral ischemia-reperfusion injury followed by contralateral nephrectomy model in mice.
The Renal ischemia-reperfusion injury model (IRI) is achieved by applying ischemia on the kidney to induce acute kidney injury by clamping the renal artery and vein. CD-1 mice undergo renal pedicle clamping for 35-40 min followed by contralateral nephrectomy 7 days after the injury. Early post-injury tubular damage as well as post-injury fibrosis, are highly consistent using this model.
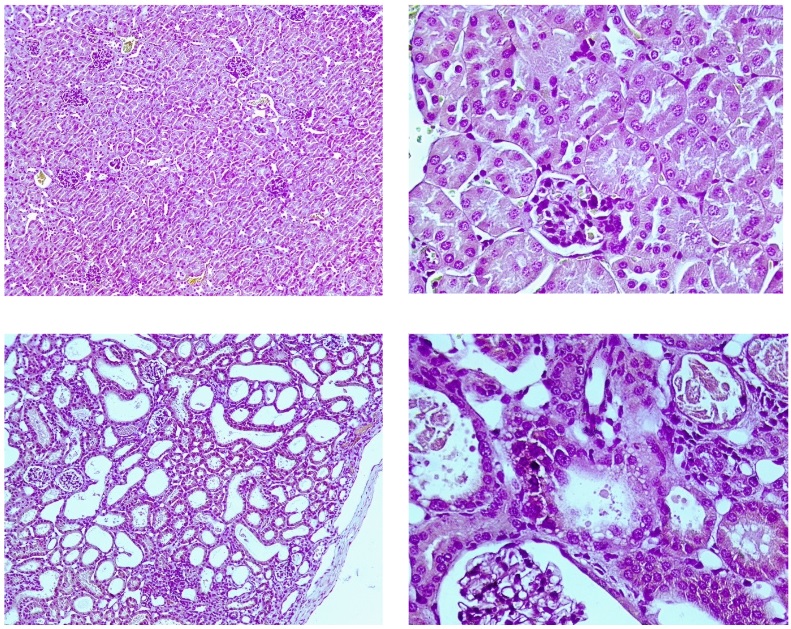
Sham-operated animals (top) have mild loss of brush border, occasional necrotic loci, occasional hemorrhages, mild vessel dilation, mild tubular hyperplasia, and occasional tubular basophilia, which could evidence increased blood supply and renal tissue regeneration. Animals underwent contralateral nephrectomy after IRI (bottom) demonstrate occasional capsule contraction or glomerulus shrinkage, mild tubular epithelium flattening, mild-to-moderate loss of brush border, mild tubular epithelium loss, occasional tubular epithelium vacuolation, occasional tubular atrophy, moderate tubular dilation, severe interstitial nephritis, moderate eosinophilic cast deposits, the mild occurrence of necrotic loci, moderate connective tissue accumulation, occasional blood capillary dilation, and vessel overflow, mild tubular hyperplasia and basophilia, which could evidence tubular apparatus violation, interstitial inflammation, necrotic processes, renal parenchyma replacement with connective tissue, and renal tissue regeneration. H&E, ×100 (right), ×400 (left).
Service details: A standard study design includes at least 2 groups of male CD-1 mice (vehicle dosed and treated with tested compound), 7 days of acclimatization period, one day IRI procedure, 7 days of post-injury recovery, 1 day for contralateral nephrectomy, and 7-10 days of the observation period. Treatment with drugs is possible in periods both before the injury to reduce the development of pathological processes and after the injury to improve the recovery. Animals are observed for mortality, body weight changes, and signs of toxicity daily for all treatment and post-treatment periods. Clinical chemistry parameters of renal failure (creatinine and urea in the blood) and NGAL levels in the blood can be determined over the time of the observation period. A gross necropsy is performed on all animals at the endpoint. Specific tests, such as hematological, urinary, and blood clinical chemistry analysis, food and water intake, histopathology, etc., in combination with more definitive toxic or gross pathology endpoints, are available on request.
Background: Diet with increased content of salt (High Salt Diet, HSD) is widely used for accelerating some aging-associated pathology – gastritis, renal, neuronal failure, hypertension development in HSD-sensitive strains of mice. HSD-induced hypertension is a relatively fast model of age-related hypertension and is used to study and treat cardiovascular and other age-associated diseases. In 2-3 weeks being kept on HSD (8% NaCl) aged normotensive mice develop at least 15 mmHg systolic blood pressure elevation compared to baseline, providing a model for studying the efficacy of new cardioprotective and anti-age agents.
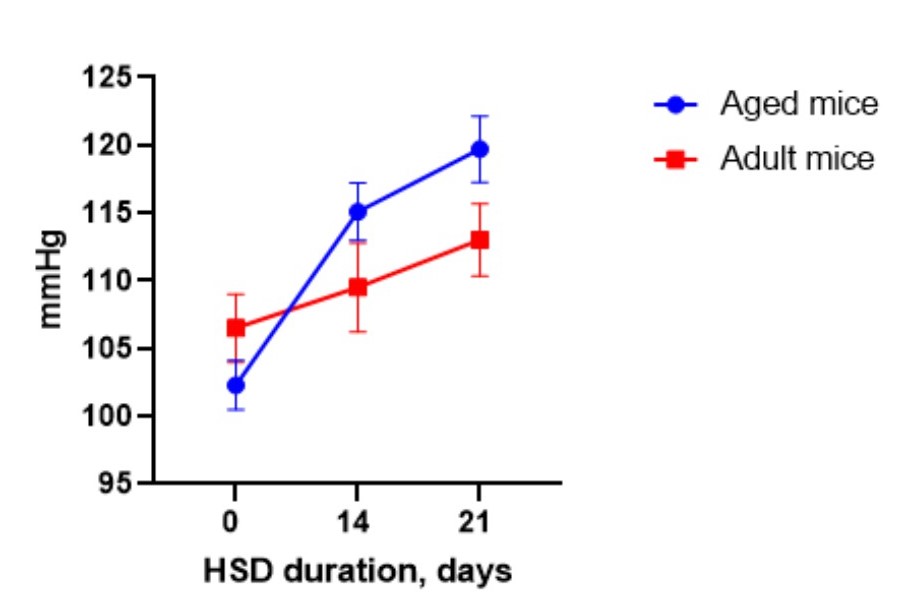
Service details: A standard study design for the model of HSD-induced hypertension includes 3 groups of mice (vehicle dosed, treated with reference compound (optional), and tested compound treated group), 10-15 mice per group, 7 days of acclimatization period, 2-3 weeks of HSD to develop hypertension, and 2-4 weeks of testing and post-treatment observation. Terms of treatment and post-treatment observation could vary. Blood pressure is measured in the tail of a mouse using volume pressure recording sensor technology with the CODA (Kent Scientific, USA) mouse/rat tail-cuff system. Mice treatment procedures include invasive (by IV, IP, SC routes), and non-invasive (PO route, and diet incorporated) routes of drug administration. Gross necropsy is performed on all animals at the endpoint. Specific tests, such as hematological, urinary, and blood clinical chemistry analysis, food intake, physiological tests (for example, grip strength test), histopathology, etc. in combination with more definitive toxic or gross pathology endpoints are available on request.
Spontaneously Hypertensive Rats (SHRs) is the best-investigated animal model of essential (or primary) hypertension used to study and treat cardiovascular disease. SH rats develop hypertension at the age of 5-6 weeks. Between 40 and 50 weeks, SHRs exhibit signs of vascular and cardiac hypertrophy, providing a classic model for studying the efficacy of new cardioprotective agents.
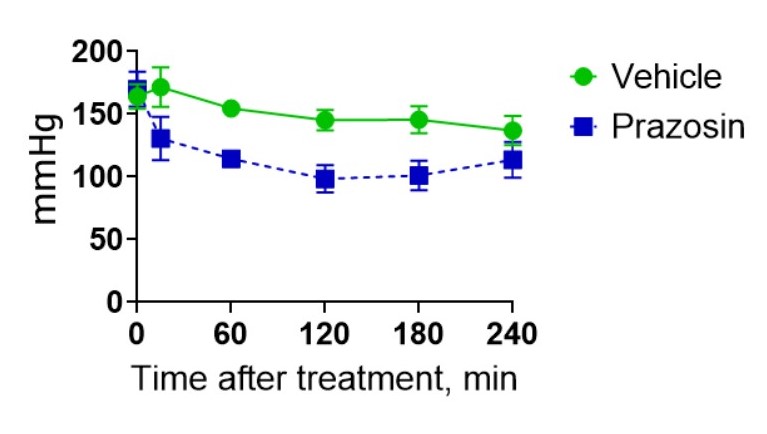
Service details: A standard study design includes at least 3 groups of rats (vehicle dosed, treated with reference compound, and tested compound treated group), 5-10 rats per group, 7 days of acclimatization period, 3-4 weeks of the treatment period, and 7 days of testing and post-treatment observation. Blood pressure is measured in the tail of a rat using volume pressure recording sensor technology with the CODA (Kent Scientific, USA) rat tail-cuff system. Rats treatment procedures include invasive (by IV, IP, SC routes), and non-invasive (PO route, and diet incorporated) routes of drug administration. Gross necropsy is performed on all animals at the endpoint. Specific tests, such as hematological, urinary, and blood clinical chemistry analysis, food and water intake, histopathology, etc. in combination with more definitive toxic or gross pathology endpoints are available on request.
Angiotensin-induced hypertension in rats
Angiotensin II (Ang II) is an important contributor to blood pressure regulation and plays a significant role in hypertension development and in the pathophysiology of all the related processes and complications. Ang II infusion causes immediate systemic hypertension in normotensive rats, which provides a suitable short-term model to evaluate antihypertensive activities of the drugs.
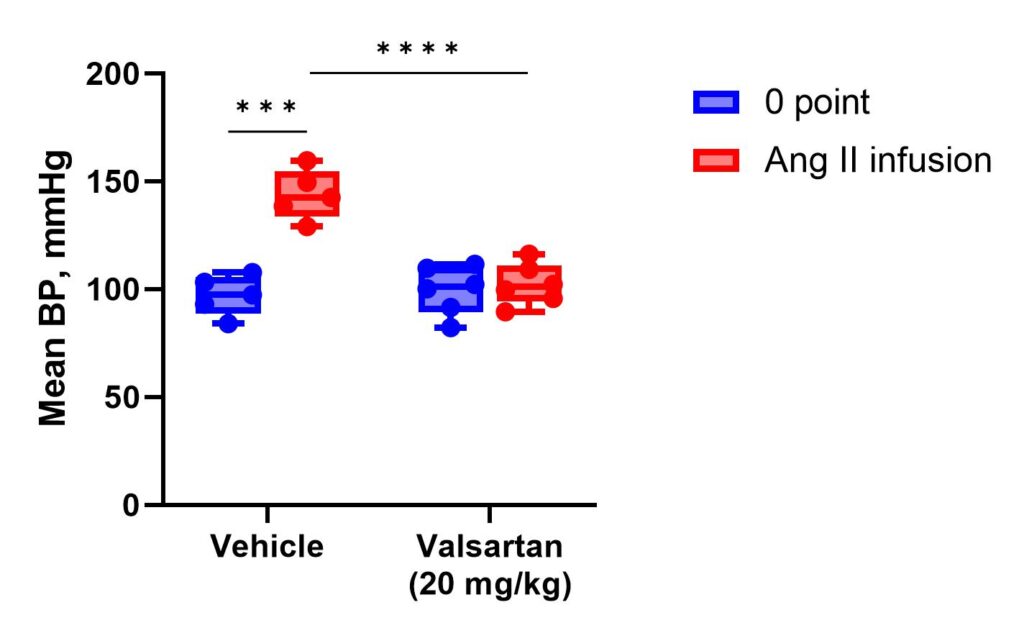
The mean blood pressure (BP) in male Wistar rats subjected to Ang II infusion (500 ng/kg/min) after single PO administration of vehicle (10% DMSO) or Valsartan at a dose of 20 mg/kg compared to baseline. Rats are placed in soft holders setting on the warming platform for 10 min to reduce stress and achieve a tail temperature of 32-34°C before the testing. Simultaneously, Ang II infusion is performed through jugular vein catheter. Systolic and diastolic BP are recorded. Mean BP and pulse pressure (difference between systolic and diastolic BP) values are then calculated.
Service details: A standard study design includes at least 3 groups of rats (vehicle-dosed, treated with reference compound, and tested compound treated group), 7 days of acclimatization period, 3 days for jugular vein catheter installation and subsequent animal recovery, and 1 day of measurements. Depending on the tested compound’s properties, animals’ treatment before the final measurements can continue up to 7 days or as required. Blood pressure is measured in the tail of a rat using volume pressure recording sensor technology with the CODA (Kent Scientific, USA) rat tail-cuff system. Rats treatment procedures include invasive (by IV, IP, SC routes), and non-invasive (PO route, and diet incorporated) routes of drug administration. Gross necropsy is performed on all animals at the endpoint. Specific tests, such as hematological, urinary, and blood clinical chemistry analysis, food and water intake, histopathology, etc. in combination with more definitive toxic or gross pathology endpoints are available on request.
Streptozotocin-induced Type 1 Diabetes
Type 1 Diabetes (T1D) is a deficiency in insulin production caused by a loss of insulin-producing β-cells of the pancreatic islets. Non-genetic animal models of diabetes are superior to the genetically determined ones because of their translatability to the clinic. T1D can be modeled by treating experimental animals (rats or mice) with Streptozotocin (STZ) has preferential toxicity toward pancreatic β-cells, which causes a reduction in insulin production, decreased levels of circulating insulin, and elevated blood glucose levels. Eventually, animals develop various diabetes-associated pathologies, such as diabetic nephropathy, cardiomyopathy, diabetic ulcers, etc. STZ-induced experimental model of T1D can be used for efficacy studies of anti-diabetic drugs, including agents acting on insulin-independent pathways and prevention of the accompanying pathology development.
Streptozotocin/High Fat Diet-induced Type 2 Diabetes
Unlike type 1 diabetes, type 2 diabetes (T2D) is associated with resistance to insulin action. In order to develop the animal pathology model, the high-fat diet (HFD) is often used. HFD leads to the development of obesity, metabolic syndrome, and a decrease in insulin sensitivity in mice, in contrast to the low-fat diet (LFD). The combination of HFD and a single Streptozotocin (STZ) injection results in metabolic changes to distinguish Type 2 Diabetes, including peripheral insulin resistance and pancreas β-cell impairment. HFD/STZ-induced model of experimental T2D can be used for efficacy testing of anti-diabetic and antiobesity drugs. The induction of disease can be carried out in rats or mice. The model is suitable for the efficacy study of the drugs stimulating the production/release of insulin from β-cells, as well as drugs reducing the production of glucose in the liver, improving tissues’ sensitivity to insulin, regulating the production of incretins, etc.
Glucose Tolerance Test (GTT)
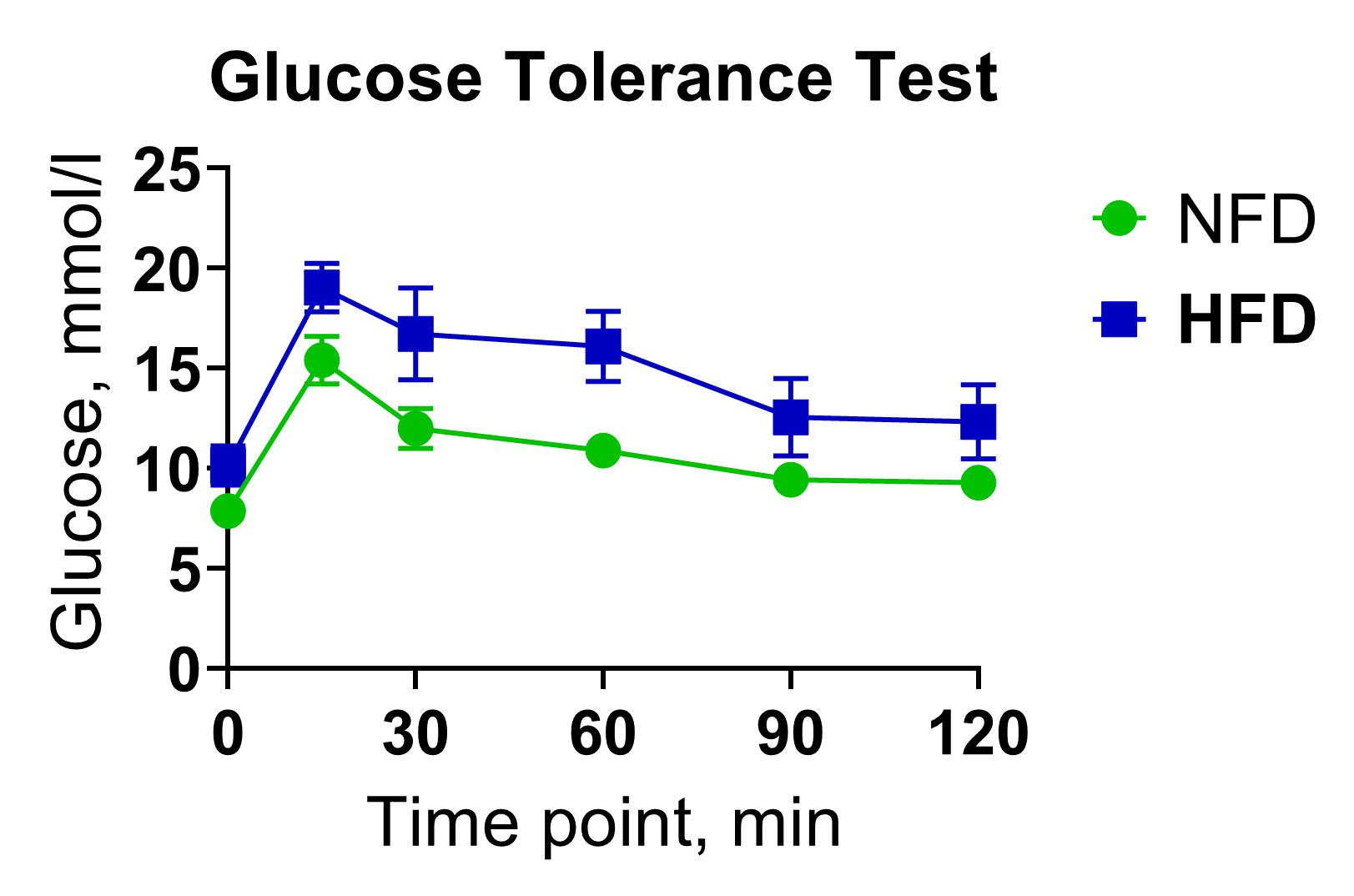
The glucose tolerance test measures the clearance rate of high glucose from organisms. GTT can be used for rapid pharmacological screening of anti-diabetic drug candidates in rodents by assessing the hypoglycemic activity of test substances following acute or chronic administration.
Insulin Tolerance Test (ITT)
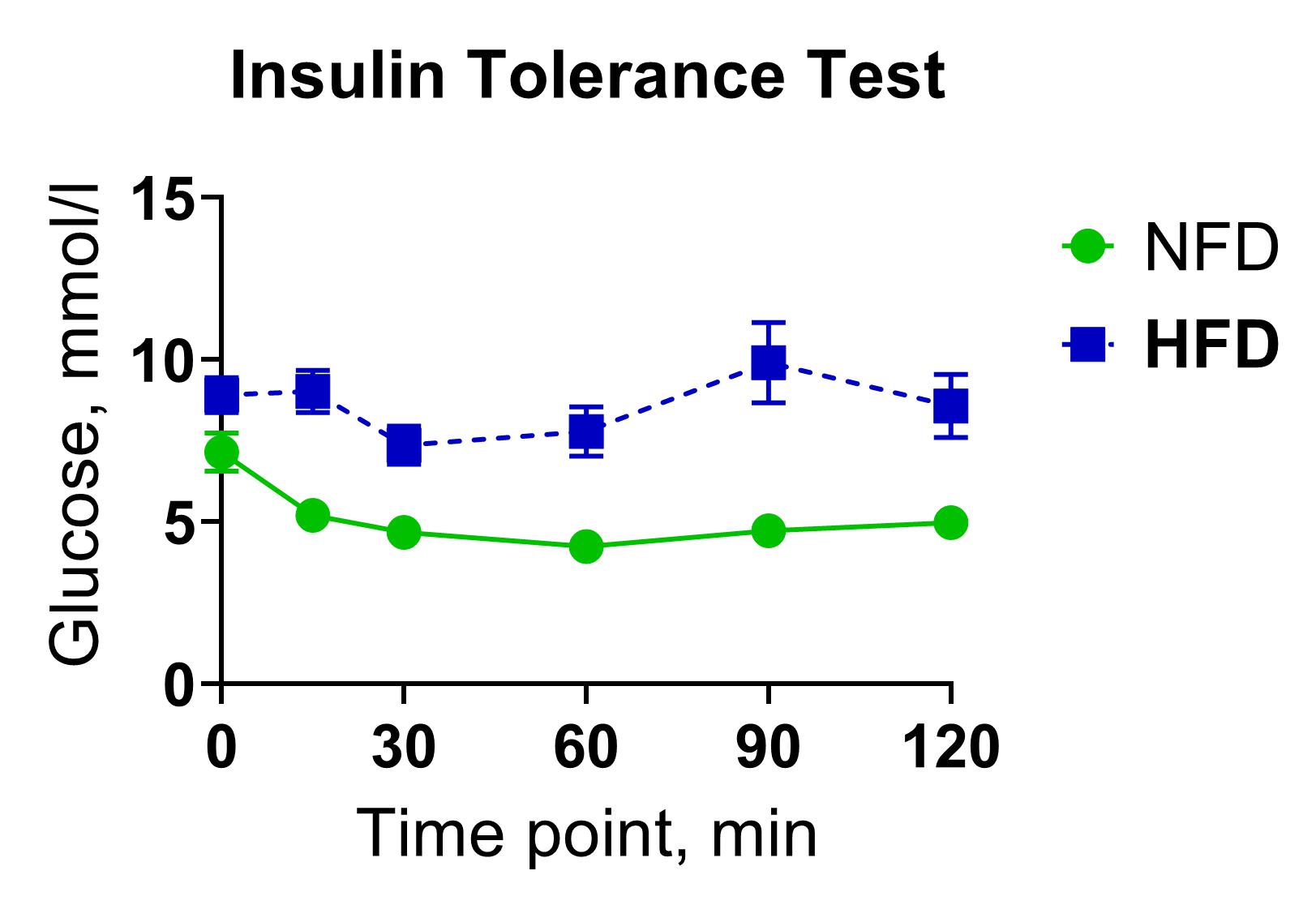
The insulin tolerance test (ITT) is a straightforward method for evaluating the sensitivity of insulin receptors in tissues by measuring blood glucose levels in circulation before and after bolus insulin injection. This technique can be used for rapid pharmacological screening of insulin-like drug candidates or insulin sensitivity modulators by assessing their hypoglycemic activity following acute or chronic administration. In a typical ITT experiment, bolus intraperitoneal injections of human insulin (0.75 U/kg) in a group of five C57BL/6N mice are used to induce hypoglycemia.
Obesity-induced Type 2 Diabetes
Obesity is associated with the development of many diseases, and the most common one among them is Type 2 Diabetes (T2D). The obesity-induced experimental model of T2D is characterized by significant weight gain, increased fasting glucose, insulin resistance, and a number of metabolic disorders, such as abnormal lipid metabolism, cardiovascular complications, nephropathy, etc. The obesity-induced T2D model C57BL/6J mice demonstrates good reproducibility and reliability of the obtained results. The model is generally used for pharmacological screening of anti-diabetic and anti-obesity drugs, as well as for the study of drugs intended for the treatment of diabetes and obesity side effects: diabetic nephropathy, cardiomyopathy, etc.
Background:
Cytochrome P450 (CYP) enzymes represent a heme containing protein superfamily metabolizing a broad variety of xenobiotics, including drugs and toxic chemicals. 11 CYP families are expressed in a human liver and gastrointestinal tract (CYP1A2, CYP2A6, CYP2B6, CYP2C8/9/18/19, CYP2D6, CYP2E1, and CYP3A4/5). Five major isoforms (CYPs 1A2, 3A4, 2C9, 2C19, and 2D6) are involved in about 95% of the known drug metabolism. Recent studies showed the increasing role of CYP2C8 and CYP2B6 in metabolism of numerous drugs and important endogenous compounds. Cytochrome P450s are of critical importance due to the two of the most significant problems in clinical pharmacology: metabolism-mediated drug-drug interactions (DDI) and individual variability in drug metabolism. It is important to evaluate the potential inhibition of a new drug candidate for the most clinically relevant CYP450 enzymes. CYP450 inhibition may potentially lead to elevated in vivo plasma levels of a co-administered drug metabolized by the inhibited enzyme, and, consequently, to adverse drug reactions and toxicity. Assessment of the following CYP450 inhibition by a new drug candidate is recommended by FDA and EMA: CYP3A4, CYP2C9, CYP2C19, CYP2D6, CYP1A2, CYP2C8 and CYP2B6.
Advantages of LC-MS/MS method over fluorimetric assay:
- LC-MS/MS analysis method is sensitive and specific, therefore human liver microsomes can be used, the fluorescent probes are not isoform-specific and should be used only with individual recombinant cytochromes
- interference can occur from the test compound exhibiting fluorescence or fluorescence quenching and lead to false results
The service is available both in panel of 5, comprising the most clinically important CYPs (1A2, 3A4, 2C9, 2C19, and 2D6), and expanded panel of 7 (1A2, 3A4, 2C9, 2C19, 2D6, 2B6, and 2C8) formats. Both individual recombinant cytochromes and human liver microsomes can be used. For the rough estimate, single point assays are typically performed for each compound at 10 µM concentration or another concentration stipulated by the customer. All test points are performed in duplicates. If a noticeable inhibition is detected, the IC50 values (test compound concentration which produces 50% inhibition) can be determined upon request. For this purpose, dose-response inhibition curves (8 points, 3-fold serial dilution) of the test compound and reference inhibitor are built starting at 100 µM concentration. The IC50 values are calculated using Microsoft Excel and GraphPad Prism software. Reference inhibitors specific for each CYP enzyme are used to assess inhibition in the control experiments for every batch of tested compounds. Test concentrations of the reference compounds correspond to approximately 5x- fold of IC50 values for the corresponding cytochromes P450, which is expected to produce 80-100% inhibition in the properly performing assay.


Deliverable:
either single point assay data for each compound at 10 µM concentration or IC50 values for the tested compounds based on 8-point, 3-fold serial dilution dose-response inhibition curves (upon request). Full study report is provided.
Sample Submission:
either single point assay data for each compound at 10 µM concentration or IC50 values for the tested compounds based on 8-point, 3-fold serial dilution dose-response inhibition curves (upon request). Full study report is provided.
Background:
Cytochrome P450 (CYP) enzymes represent a heme containing protein superfamily metabolizing a broad variety of xenobiotics, including drugs and toxic chemicals. Eleven CYP families are expressed in human liver and gastrointestinal tract (CYP1A2, CYP2A6, CYP2B6, CYP2C8/9/18/19, CYP2D6, CYP2E1, and CYP3A4/5), and 5 of them (CYPs 1A2, 2C9, 2C19, 2D6 and 3A4) are involved in about 95% of the known drug metabolism. Cytochrome P450s are of critical importance due to the two of the most significant issues in clinical pharmacology: metabolism-mediated drug-drug interactions and individual variability in drug metabolism (CYP450 gene polymorphism).
CYP450 reaction phenotyping involves the identification of enzymes responsible for metabolism of the test article, which is useful for prediction of drug-drug interactions (DDI) and is recommended by FDA. In case when a new drug candidate is metabolized by more than one isozyme, blocking one metabolic pathway by coadministered inhibiting drug engender metabolic switching to an alternative uninhibited enzyme.
Service Details: In this assay, two metabolically active test systems are used: individual human recombinant cytochromes (CYPs 1A2, 2C9, 2C19, 2D6 and 3A4) and human liver microsomes. The test compound is incubated with each CYP450 isoform and the metabolising ability of the enzyme is estimated by disappearance rate of parent drug using HPLC-MS/MS analysis. Reference cytochrome-specific substrates are used as controls. In the microsomal assay the isoform-specific CYP450 inhibitors are used, and the increase of the half-life (t1/2) in the presence of the inhibitor indicates which enzyme is responsible for the metabolism of the compound.
Deliverable: Metabolic stability data of 1 test article and 5 reference compounds (Phenacetin, Testosterone, Diclofenac, Omeprazole, and Dextromethorphan) in the presence of individual human recombinant cytochromes at tree time points over 60 minutes. Metabolic stability data for 1 test article in human liver microsomes at the presence of specific cytochrome inhibitors responsible for its metabolism (identified in the study with recombinant enzymes) at five time points over 60 minutes obtained by HPLC-MS/MS analysis. Data include parent compound percent remaining, half-life and clearance values. Full study report is provided.
Sample Submission: A minimal accurately weighable quantity of dry compound (~1 mg or 2 µmol) or 50 µL of 20 mM stock DMSO solution is required for this assay. For multiple assays, lesser amount of compound per assay may be sufficient, depending on the particular project. We do not need to know structures of the molecules for ADME testing. However, brutto formulas have to be provided for all studies involving MS detection.
Background and service details: Determination of stability of the potential drugs in plasma is indispensable in early stages of the drug discovery process, as it is crucial for pharmacokinetic readouts and has direct impact on in vivo efficacy. Certain classes of drug molecules, such as those containing ester or amide-linked groups are prone to enzymatic hydrolysis by plasma esterases, amidases or proteases. On the other hand, enzymatic activation of some prodrugs that takes place in plasma is essential for their function. Hence, plasma enzymes can significantly alter the bioavailability of the active compounds, and therefore determination of the compound stability in plasma has both pharmacokinetic and clinical significance.
Incubations are carried out in 96-well polypropylene plates in 5 aliquots of 70 μL each (one for each time point). Test compounds and reference compounds are incubated at 37°C. Five time points over 120 minutes are analyzed (0, 20, 40, 60 and 120 min). All tests are performed in duplicates. The samples are analyzed by HPLC-MS (API3000, AB Sciex).
Deliverable
The percentage of parent compound remaining in plasma after incubation is plotted versus incubation time; plasma half-life (T½) is calculated from the obtained curve. Full study report is provided.
Sample Requirement/Submission
A minimal accurately weighable quantity of dry compound (~1 mg or 2 µmol) or 50 µL of 10-20 mM stock DMSO solution is required for this assay. For multiple assays, lesser amount of compound per assay may be sufficient, depending on the particular project. We do not need to know structures of the molecules for ADME testing. However, brutto formulas have to be provided for all studies involving MS detection.
Background: At early drug discovery stages it is critical to rapidly evaluate the metabolic stability of many new chemical entities. Single time point microsomal stability pre-screen assay, focused on stability ranking of large compound sets, allows increasing throughput and reducing costs. Moderate incubation time used in this assay (e.g. 15 min for mouse liver microsomes) provides valid metabolic stability evaluations for both unstable and stable compounds, which are in good agreement with those derived from the standard multi time point experiments.
Service Details: Single time point microsomal stability pre-screen is a 96-well plate automated assay performed on sets starting from 30 compounds. Test compounds are incubated with microsomes under the standard hepatic microsomal stability assay conditions. The parent compound loss is evaluated by LC-MS/MS measurements (API5000 detector, AB Sciex). This service also includes incubation of two control drugs and a control without co-factors.
Deliverable: Data include parent compound percent remaining. Full study report is provided.
Sample Submission: A minimal accurately weighable quantity of dry compound (~1 mg or 2 µmol) or 50 µL of 10-20 mM stock DMSO solution is required for this assay. For multiple assays, lesser amount of compound per assay may be sufficient, which should be discussed for each particular project. We do not need to know structures of the molecules for ADME testing. However, we ask our customers to provide brutto formulas for all studies involving MS detection.
Background and Service Details::
Liver is a primary site of drug metabolism, and drug metabolic transformations may have significant impact on its efficacy and safety. For this reason, drug candidates are screened early in the discovery process for metabolic stability. Microsomes from human or animal liver are useful models to quickly and inexpensively predict hepatic clearance in vitro for the corresponding species. Stability experiments can be done either with hepatic microsomal fraction to investigate only the cytochromes P450-mediated (Phase I) metabolism or with the S9 fraction, which consists of both hepatic microsomes and cytosol. The advantage of using S9 fraction is that it contains both Phase and Phase II enzymes and can be used to investigate Phase II metabolic pathways in vitro, when supplemented with the corresponding cofactors such as UDPGA (for glucuronidation) and PAPS (for sulphation).
Metabolic stability assays are typically performed using mouse, rat, or human microsomes or S9 fraction (microsomes from other species are available upon request). Test compounds are incubated with microsomes supplemented with cofactors at 37°C. Typical conditions are the compound concentration of 2 µM and 5 sampling time points over 40 min, in two independent replicates. At each time point, the reactions are terminated with acetonitrile. The samples are centrifuged and the relative parent compound concentrations are evaluated by LC-MS/MS. The incubation of two control drugs with microsomes and blank control reaction without co-factors are used as controls.
Deliverable:
Data include parent compound percent remaining, half-life (t1/2), and intrinsic clearance (Clint) values. Full study report is provided.
Sample Submission:
A minimal accurately weighable quantity of dry compound (~1 mg or 2 µmol) or 50 µL of 10-20 mM stock DMSO solution is required for this assay. For multiple assays, lesser amount of compound per assay may be sufficient, depending on the particular project. We do not need to know structures of the molecules for ADME testing. However, brutto formulas have to be provided for all studies involving MS detection
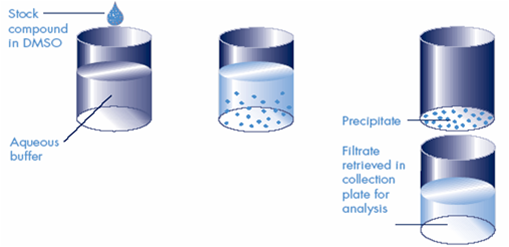
Determining compound solubility is essential for the early stages of the drug discovery process and lead optimization. Low solubility can lead to unpredictable and unreliable results during in vitro testing, thereby increasing the development costs. Solubility issues at the later stages of the drug discovery may lead to poor bioavailability, underestimated toxicity, and other obstacles, lowering the chances of a given drug candidate for success. Solubility can be measured either as a kinetic or thermodynamic value. Typically, the kinetic solubility method is used for early-stage drug discovery, as it is fast and well-suited for the HTS format. In this case, solid compounds are first dissolved in DMSO. Then linear serial dilutions of each compound are added to an aqueous buffer and observed for precipitate formation when the compound is not completely soluble. Precipitate appearance can be evaluated by light scattering (laser nephelometry method). For better precision, the solution can be subjected to high-speed centrifugation or filtration using special solubility filter plates. Then the compound concentration is measured in the saturated solution directly by UV or LC-MS using separately built calibration curves. Thermodynamic solubility is important for lead optimization and drug formulation stages. It is usually determined for pure compounds: crystalline powders, amorphous substances, and liquids. In this modification of the solubility assay, long (24 hours or more) incubations are required. Measurements are usually performed by the shake flask method with UV-Vis or LC-MS detection.
Deliverable: Full study report is provided, including solubilities and calibration curves for test and reference compounds (an example report is shown).
Sample Submission: Sample requirements are at least 50 µL of 20 mM stock compound solutions in DMSO for kinetic solubility measurements and 2 x 4 µmol of dry compound for thermodynamic solubility measurements. We do not need to know the structures of the molecules for ADME testing. However, brutto formulas must be provided for all studies involving MS detection.
Background
Laser Nephelometry is a high-throughput and affordable option for rapid evaluation of kinetic solubility for a large number of compounds in any aqueous buffer. Linear serial dilutions of each compound to be analyzed are prepared from DMSO stock solutions in an aqueous buffer of the specified composition and pH and the precipitation points for each compound are determined from the light scatter graphs (typical working solubility range is from 50-100 µM up to 1-2 mM). This assay is typically performed in duplicates.
For a rough estimate of aqueous solubility (“solubility not lower than…”), this method can be even more simplified: instead of linear serial dilutions, light scattering can be measured in two concentrations of compounds (high-throughput solubility threshold method). Those compounds yielding light scattering signal values higher than that of the highly soluble compound 2′-Deoxy-5-fluorouridine, are considered insoluble at tested concentrations. As a control, reference compound poorly soluble at the same concentrations is used.
Deliverable
Compound’s solubility normalized to deoxyfluorouridine (100% soluble) and raloxifene (0% solubility) is calculated. Selected ranges of Relative Solubility (>0.8, 0.6-0.8 and <0.6) allow dividing compounds in clearly distinguishable groups (good solubility, intermediate solubility, and poor solubility) at the chosen solubility threshold. Full study report is provided.
Sample Submission
Typically, a minimal accurately weighable quantity of dry compound (~1 mg or 2 µmol) or 50 µL of 20 mM stock DMSO solution, is required for this assay. However, the exact required amount would depend upon the solubility range of interest.
List of references:
- T.A. Fligge, A. Schuler, Integration of a rapid automated solubility classification into early validation of hits obtained by high throughput screening. Journal of Pharmaceutical and Biomedical Analysis, 2006, Vol. 42, P. 449–454.
- 2. M.E. Stevens, P.J. Bouchard, I. Kariv, T.D.Y. Chung, K.R. Oldenburg, Comparison of automation Equipment in high throughput screening. Journal of Biomolecular Screening, 1998, V.3, #4, P 305-311
- 3. C.D. Bevan, R.S. Lloyd, A High-Throughput Screening Method for the Determination of Aqueous Drug Solubility Using Laser Nephelometry in Microtiter Plates. Anal. Chem. 2000, V. 72, P. 1781-1787.
Background
Stability in aqueous solutions is a fundamental requirement to a successful drug candidate. Degradation may be caused by a variety of mechanisms: hydrolysis, oxidation, light-catalysed degradation and others. At early stages of drug discovery, screening for stability in buffer solutions at acidic, neutral and basic pH is desirable for eliminating potentially troublesome candidates.
Chemical stability analyses are often performed by HPLC with UV-Vis detection at 3 wavelengths. Yet, in some cases degradation products may be poorly separated from parent compounds, causing inaccuracy in analysis. For this reason in our lab this analysis is typically performed using LC/MS (although UV-Vis detection can also be performed upon request).
The assay is performed in a reusable 96-well Teflon plate (Millipore) to avoid possible artifacts caused by adsorption of certain compounds to polypropylene surfaces. Upon request, polypropylene plates can be used in the same assay setup for the non-specific binding assessment, which can be conveniently combined with the chemical stability assay, as PTFE (Teflon®) and polypropylene have different binding characteristics.
Deliverable: Stability is calculated as % test compound remaining relative to the T=0 peak area. Full study report is provided.
Sample Submission: A minimal accurately weighable quantity of dry compound (~1 mg or 2 µmol) or 50 µL of 10-20 mM stock DMSO solution is required for this assay. For multiple assays, lesser amount of compound per assay may be sufficient, depending on the particular project. We do not need to know structures of the molecules for ADME testing. However, brutto formulas have to be provided for all studies involving MS detection.
A certain balance of lipophilicity and hydrophilicity is required for a successful drug candidate. Water solubility is essential for a drug to be dissolved in plasma and other aqueous biological fluids, whereas lipophilicity is indispensable for penetrating through biological membranes and is of high importance for the compounds aimed for oral dosing. A widely used model to access the physicochemical properties of the compound is LogP (partition coefficient). Lipophilicity is typically accessed as the distribution of the tested compound between two solvents – typically non-aqueous organic (n-octanol) and aqueous (pH-buffered water), and then LogP is expressed as a log10 of the concentration ratio between two phases. LogP is widely used in cheminformatics and is a component of Lipinski’s “rule of five”, which is a golden standard to evaluate a drug-likeness of a compound. According to this rule, the successful drug candidate should possess a LogP value not greater than 5.
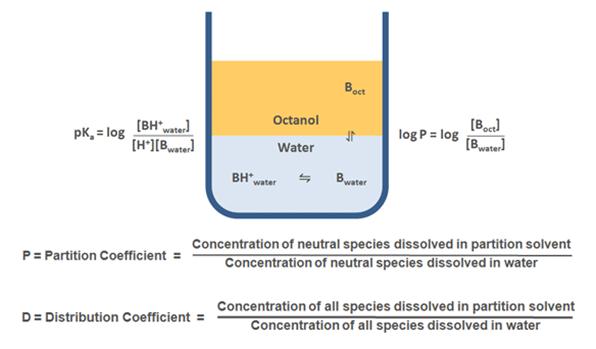
LogD is a distribution coefficient widely used to measure the lipophilicity of ionizable compounds, where the partition is a function of the pH. For non-ionizable compounds LogP = LogD throughout pH range, whereas for ionizable compounds LogD takes into account the partition of both ionized and non-ionized forms. LogD is more convenient for practical measurements, as it takes into account solution pH, which is important for the analysis of the drug candidate properties in various biologic media with different pH values.
Note: Pricing for this service refers to logD determination at one selected pH. For non-charged compounds (at the pH used for logD measurements) logD equals logP, and there is no extra charge for logP measurement. For compounds with known pKa values, price for logP determination will be equal to the price of logD determination; the same applies for the cases where in silico calculated pKa values are used. Pricing for the experimental pKa measurements is subject to discussion and depends on the properties of the tested compounds.
Deliverable
Calculations of the partition ratios (peak area of the analyte in octanol phase to the peak area of the analyte in PBS buffer). A full study report is provided.
Sample Requirement/Submission
A minimal, accurately weighable quantity of dry compound (~1 mg or 2 µmol) or 50 µL of 10-20 mM stock DMSO solution is required for this assay. For multiple assays, a lesser amount of compound per assay may be sufficient, depending on the particular project. We do not need to know the structures of the molecules for ADME testing. However, brutto formulas have to be provided for all studies involving MS detection.
LogD/LogP is one of our ADME tests for physicochemical properties evaluation:
- Aqueous Solubility
- Shake flask solubility using Millipore or pION Solubility Filter Plates (UV or MS-MS detection)
- Laser nephelometry
- Chemical Stability Assay
- Determining pKa
Background:
The tendency of a compound to donate a proton is expressed as its acid ionization constant (dissociation constant), or Ka, and is usually represented as pKa (pKa = ̶ log Ka). There is a documented correlation between pKa of a drug and its solubility that is widely used for the prediction of drug behavior in vivo. pKa value may have a profound effect on pharmacological activity, bioavailability and toxicity of a drug, and therefore is a valuable readout in the selection process.
Deliverable:
Final report comprising information on the analysis, methodology, raw data, and interpretation of the results is provided.
Sample Submission:
Approximately 40 µmol (20 mg) of dry compound is necessary for this test.
Determining compound aqueous solubility is an essential tool for early stages of the drug discovery process, as well as for lead optimization. Low aqueous solubility can lead to unpredictable and unreliable results during in vitro testing, thereby increasing the development costs. Solubility issues at the later stages of the drug discovery may lead to poor bioavailability, underestimated toxicity and other obstacles, lowering the chances of a given drug candidate for success. Solubility can be measured as either a kinetic or thermodynamic value
Aqueous solubility: Kinetic study
Typically, scientists use the kinetic solubility method in early-stage drug discovery, as it is fast and well suited for HTS format. In this kind of test, solid compounds are first dissolved in DMSO and then linear serial dilutions of each compound are added to aqueous buffer and observed for precipitate formation when the compound is not completely soluble. Precipitate appearance can be evaluated by light scattering (laser nephelometry method). For better precision, the solution can be subjected to high-speed centrifugation or filtration using special solubility filter plates and then the compound concentration is measured in the saturated solution directly by UV or LC/MS using separately built calibration curves (shake-flask method).
Aqueous solubility: Thermodynamic study
Thermodynamic solubility is important for lead optimization and drug formulation stages. It is usually determined for pure compounds: crystalline powders, amorfous substances and liquids. In this modification of solubility assay long-term (12-24 hours) incubation is used. Measurements are usually performed by the shake-flask method with LC/MS detection.
Currently, the oversight of laboratory animal research in Ukraine does not involve the national government. Instead, this responsibility is delegated to local Ethics Committees. At BIENTA Ltd., the local Animal Care and Use Committee (BACUC) is entrusted with monitoring all animal research activities. The committee carries out several important tasks to ensure compliance with international animal welfare regulations and ethical standards (which one).
One of the main responsibilities of the BACUC is to review and approve Animal Use Protocols (AUPs) for studies involving laboratory rodents. They also ensure that researchers adhere to animal welfare regulations by conducting regular assessments, especially with the arrival of new projects. Additionally, the committee performs post-approval research monitoring on a regular basis.
Primary BACUC functions include:
- Monitoring compliance with sanitary standards in all areas of the animal facility.
- Initial training upon employment and subsequent regular training every year. The Committee also provides training to personnel engaged in the research and testing of rodents, either upon approval of new AUPs for new methods or regularly every three years for routine methods.
- Reviewing, approving, and suggesting corrections to each AUP according to the requirements.
The commitment of the BACUC and its members to upholding the highest standards of animal welfare and research ethics is essential for the successful implementation of animal research at BIENTA Ltd. If you have questions, please contact

BACUC Policies are based on
several international regulatory documents and guidelines that govern the use of laboratory animals for scientific research. Here are some of the key ones:
- The Guide for the Care and Use of Laboratory Animals (Guide): Published by the National Research Council (NRC).
https://grants.nih.gov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animals.pdf - European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes (ETS 123).
https://rm.coe.int/168007a67b - Directive 2010/63/EU on the protection of animals used for scientific purposes: This European Union directive provides a framework for the protection of animals used in scientific research.
https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:276:0033:0079:en:PDF - International Council for Laboratory Animal Science (ICLAS) publications: ICLAS produces various publications and guidelines related to laboratory animal science.
https://iclas.org/membership-iclas/ - International Standards Organization (ISO) standards: ISO 10993 for biological evaluation of medical devices and ISO 10993-2 for animal welfare requirements.
https://nhiso.com/wp-content/uploads/2018/05/ISO-10993-2-2006.pdf - OECD Guidance Document on the Recognition, Assessment, and Use of Clinical Signs as Humane End Points for Experimental Animals Used in Safety Evaluation, Series on Testing and Assessment, N°19, ENV/JM/MONO (2000)7, OECD, Paris. (2000).
https://read.oecd-ilibrary.org/environment/guidance-document-on-the-recognition-assessment-and-use-of-clinical-signs-as-human-endpoints-for-experimental-animals-used-in-safety-evaluation_9789264078376-en#page1 - WMA Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects
https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ - DIRECTIVE 2010/63/EU OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 22 September 2010 on the protection of animals used for scientific purposes
https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:276:0033:0079:en:PDF - Law of Ukraine “On the Protection of Animals from Cruelty.”
https://zakon.rada.gov.ua/laws/show/3447-15#Text
Behavioral Tests
Irwin Test/Functional Observational Battery (FOB) is developed for rapid in vivo screening of new neurotrophic and neurotoxic substances. The battery of behavioral observations, assess six broad categories of CNS function including excitation, sedation, stereotypy, pain/anesthesia, reflexes, and autonomic balance.
The Beam walking test was designed for the investigation of ataxia-motor dysfunction in rodents. The test is used for detecting violations of the equilibrium and coordinating movement mediated by cerebellar dysfunction. This test is used to evaluate new drug candidates for their effect on coordinating movement.
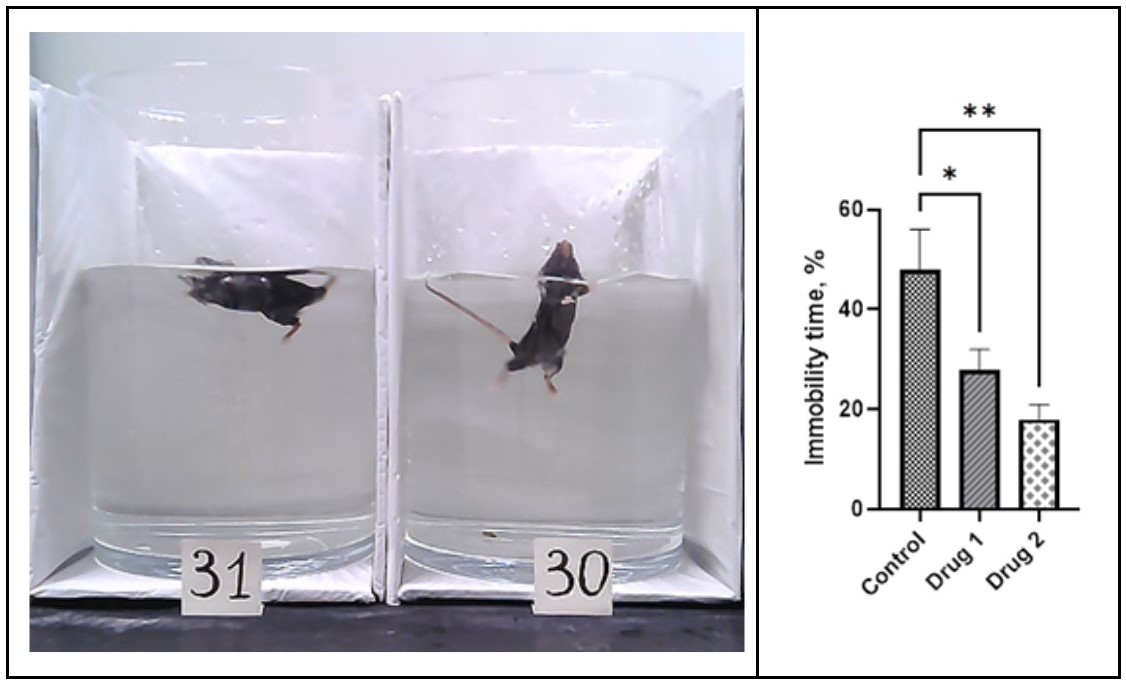
The forced swim test is the main test to assess symptoms of depression using animal models. During the test when rodents are forced to swim, we are assessing the rodent’s active or passive behavior. Active behavior characterizes by active swimming, climbing, and looking for an escape route; passive behavior characterizes by stopping swimming, “giving up the search for escape,” floating on the water’s surface without struggling and showing the minimal movements necessary to keep an animal’s head above water. We measure and score swimming/escaping (active) or passive (floating/immobility) time in the tall cylinder with water. The forced swim test is commonly used to assess the antidepressant activity of compounds. The reduction of immobility time and increase of active swimming time is interpreted as an antidepressant-like effect of the pharmacological action.
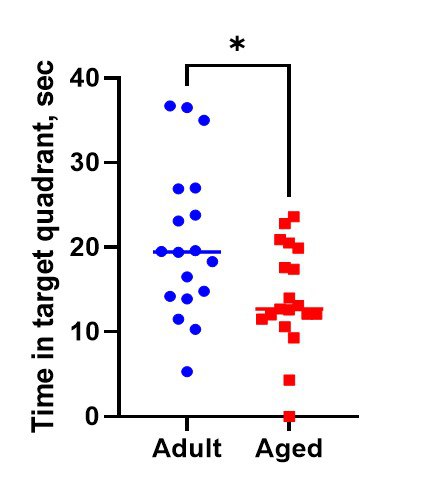
The Morris/Radial Water Maze Test is a widely used method for studying spatial learning and memory. The method is based on the animal’s (rat or mouse) trying to find the hidden underwater platform and escape swimming in the pull of water by using visual cues. Morris Water Maze is an appropriate model for studies of such pathology as Alzheimer’s disease, depression, or amnesia, and age-related changes.
Method Advantages:
- the aversive nature of the water motivates the animal to explore the territory
- rapid learning
- easy measuring of parameters
- no requirement for food deprivation
- less risk of potential confounding scent cues
- both working and reference memory can be simultaneously tested in one experiment.
Method Limitations: The animal’s ability to swim and physical condition or endurance can impact the results. The act of being immersed in water and forced to swim can induce stress that may alter the outcomes of each repeated trial.
Nociception Assays:
The Hot Plate Test allows for exploring the pain response caused by heat in rodents, as well as the influence of the centrally-acting analgesic on this process. Peripherally acting drugs are ineffective in this test.
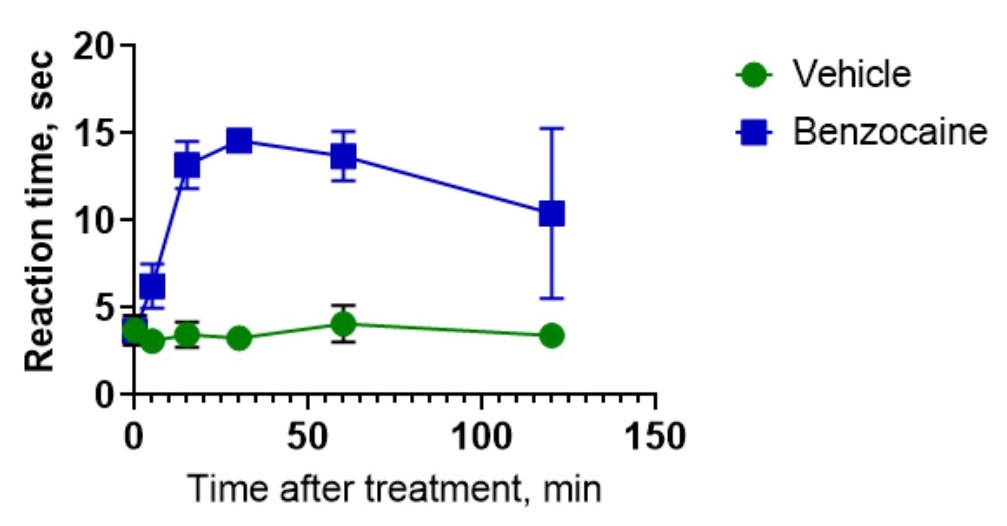
The tail-flick test is the most frequently used test for nociception in animals. Unlike the Hot plate test, the tail-flick test also allows local anesthetics to be tested. During the test, radiant heat applies to the rodent’s tail, and time is measured until the animal moves the tail away because of pain caused by the heat beam. The test set for the measurement of the nociceptive sensitivity of animals for the analgesic properties of substance assessment. Time increase before avoidance response to the thermal stimulus reveals an analgesic action.

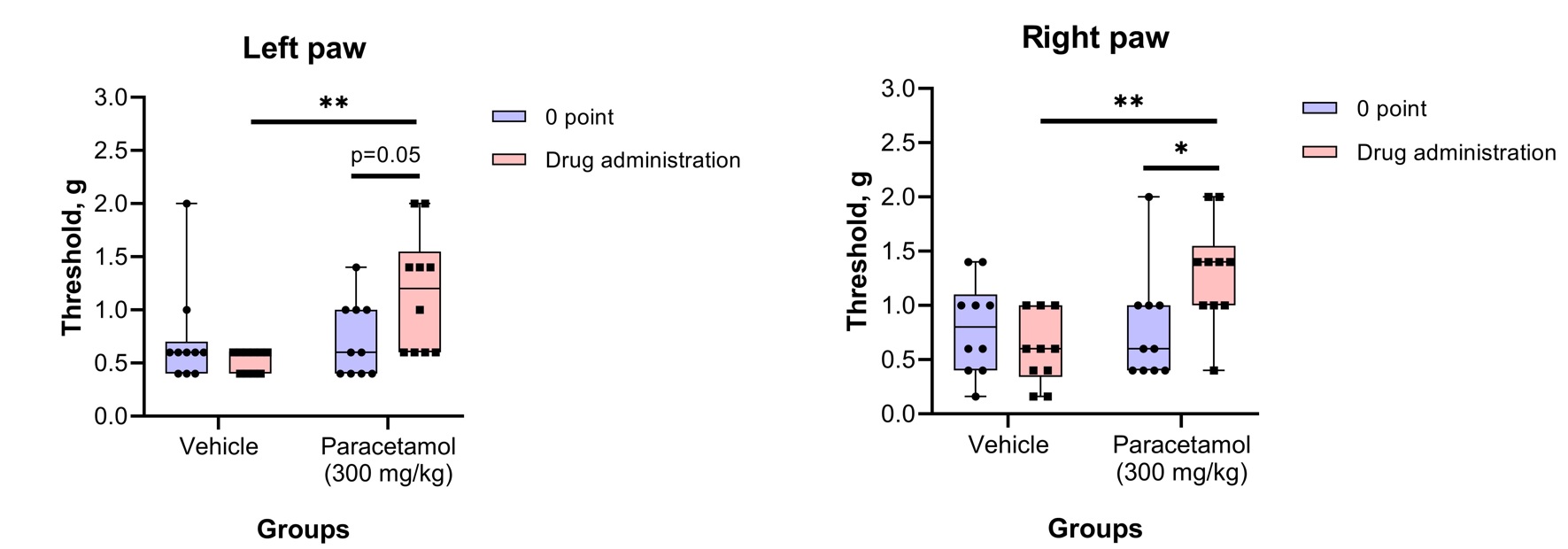
Von Frey testThe von Frey test involves applying a punctate stimulus to a given region of the rodent’s body, usually the plantar surface of the hind paw, and recording the stimulus intensity that evokes a withdrawal reflex.
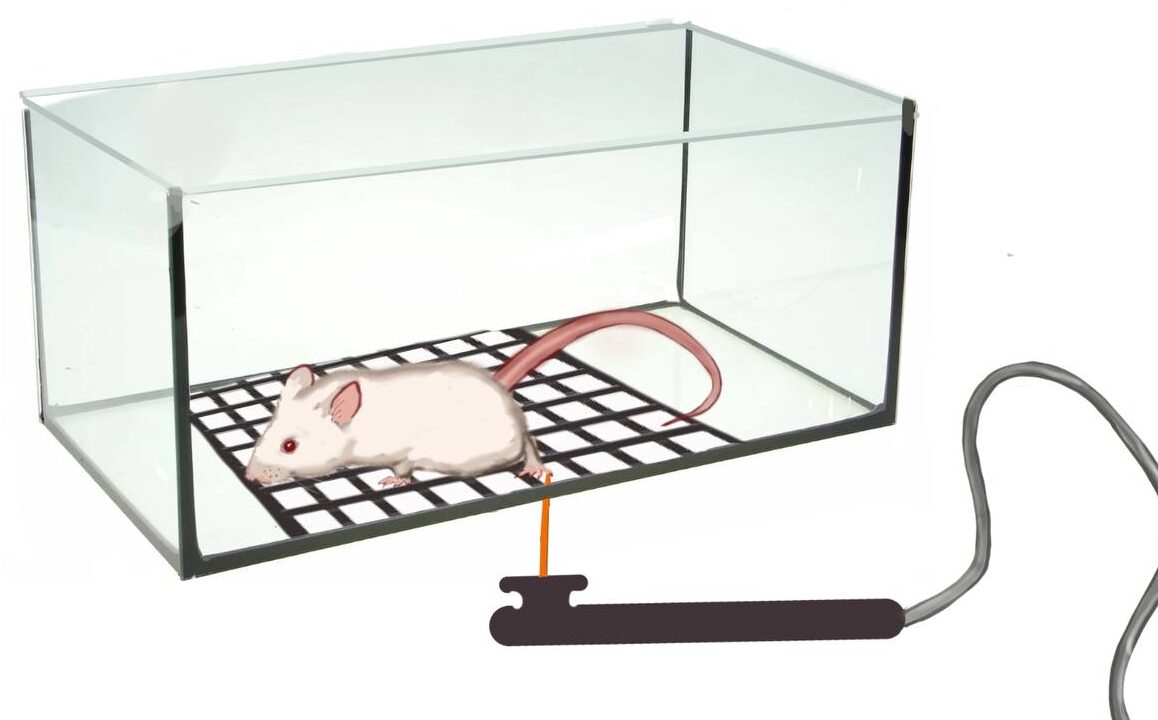
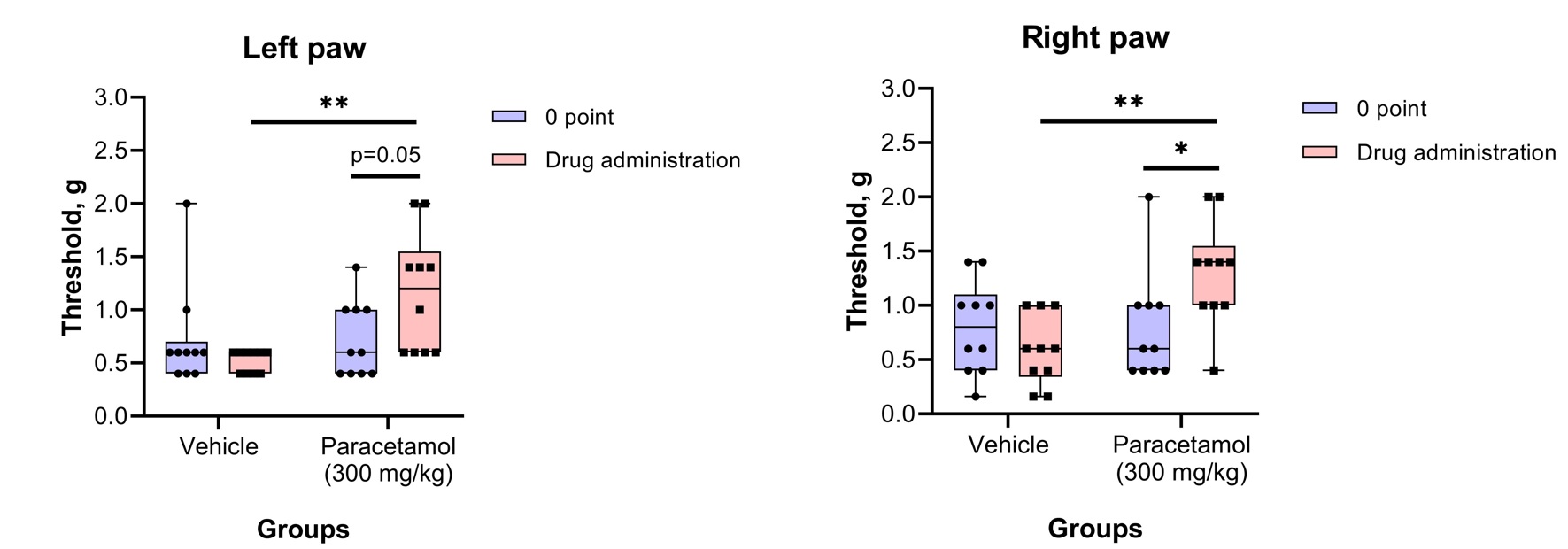
Physiological Tests:
Blood pressure (BP) in rodents is measured in the tail of the mouse or rat using volume pressure recording sensor technology with the CODA (Kent Scientific, USA) mouse/rat tail-cuff system.
The grip strength test is a non-invasive method for evaluating rodent muscle force in vivo. It allows studying the neuromuscular functions by determining the maximal peak force developed by a rodent when the operator pulls it out of a specially designed grid or bar available for both fore and hind limbs. The Grip Test is included in the Functional Observational Battery (FOB) to screen for neurobehavioral toxicity.
Rotarod test is a non-invasive method for evaluating rodent balance, physical condition, and motor coordination in vivo. It allows studying the neuromuscular functions by determining the maximal riding time (seconds) and speed at fall developed by a rodent, which naturally tries to stay on the rotating cylinder (rod) and avoid falling to the ground.