Syngeneic mouse models are established with murine cancer cell lines in immuno-competent mice. These models are excellent for studying new antitumor therapeutic agents in the presence of a functional immune system. The C57Bl/6J or Balb/c mouse strains are typically employed to establish the syngeneic model.
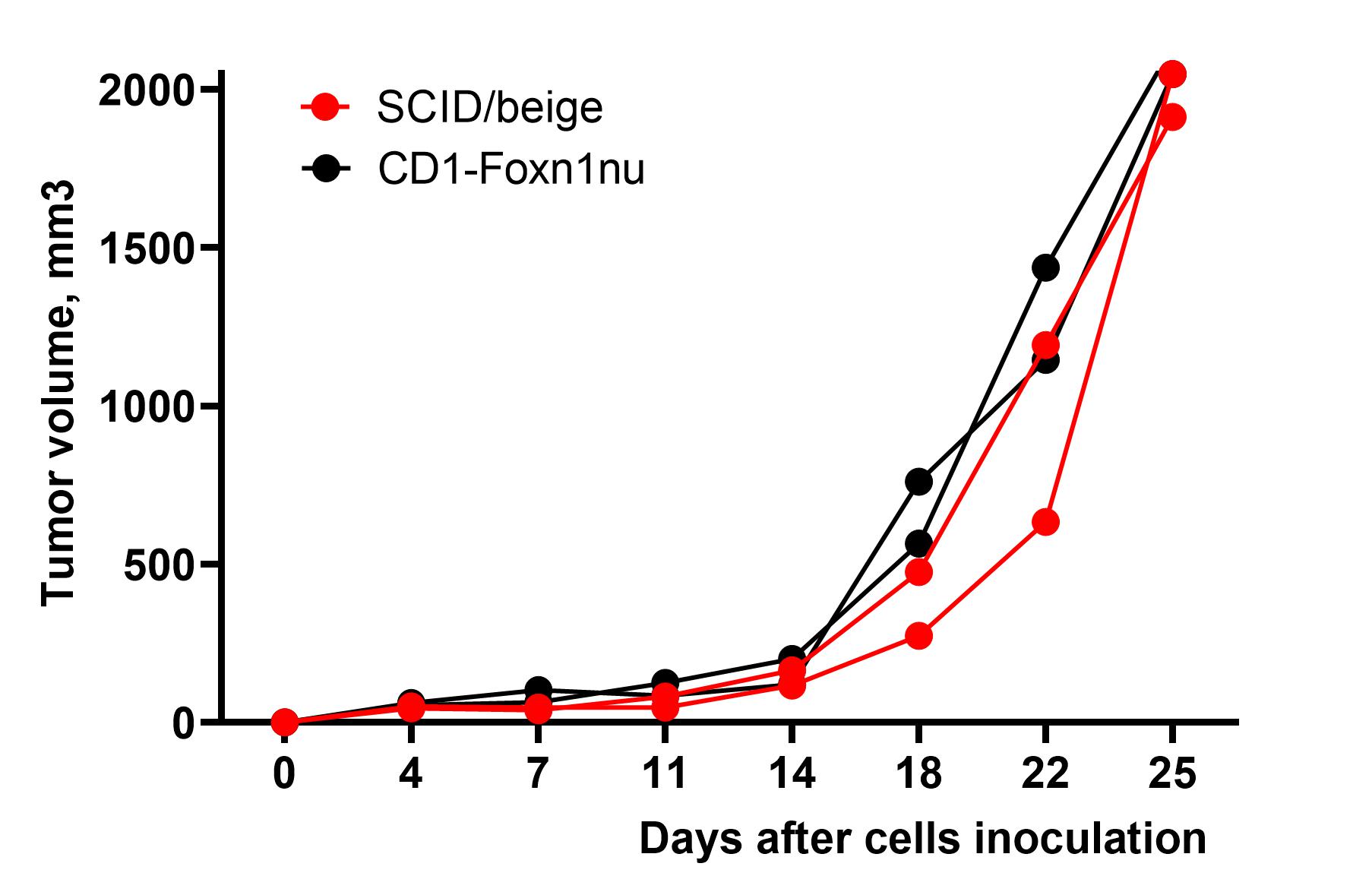
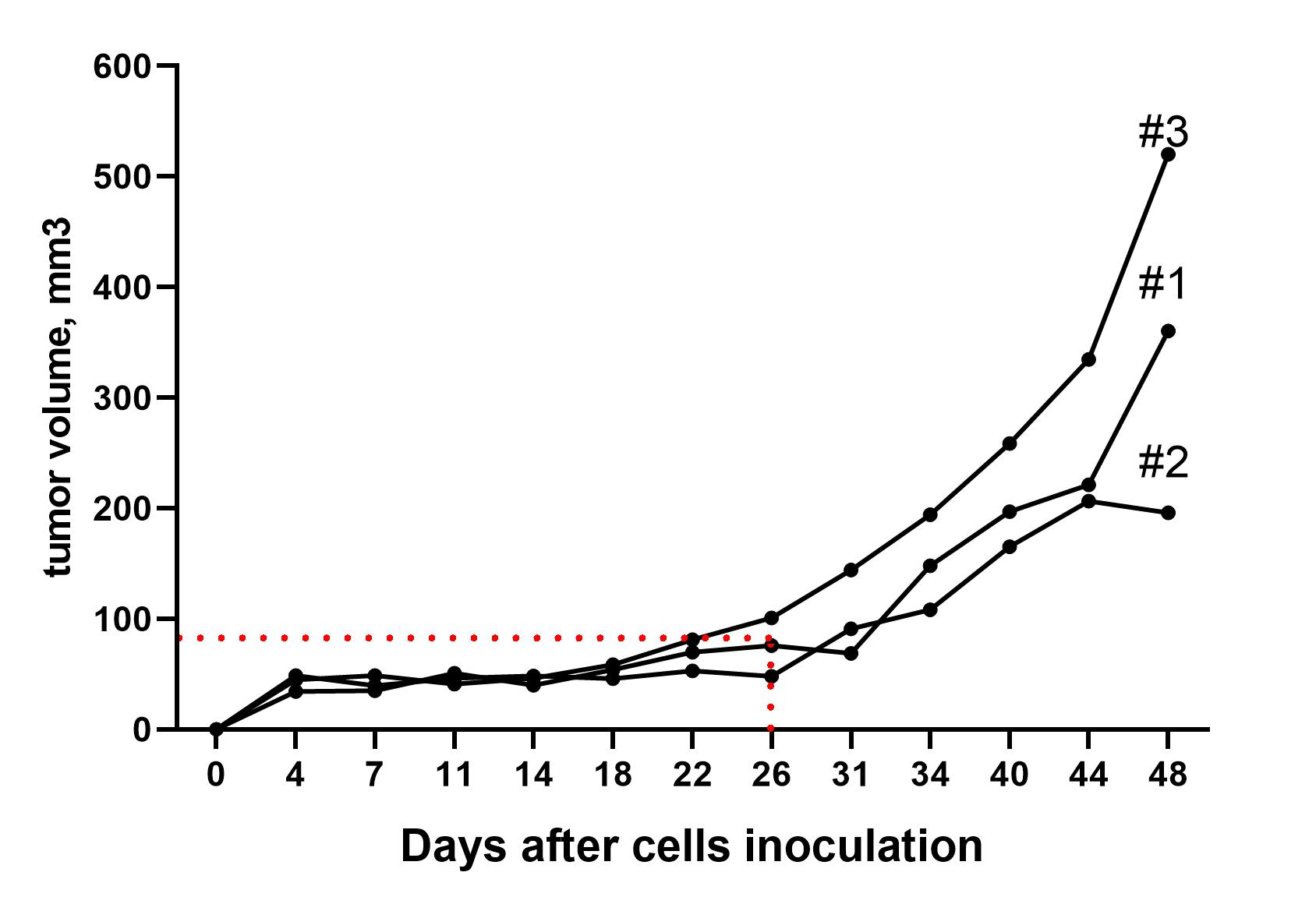
Xenograft models utilize human cancer cells that are inoculated into athymic (nude) or severe combined immune deficient (SCID) mice. These xenograft models can be developed as subcutaneous or orthotopic tumors, allowing for the evaluation of drug efficacy specifically against human cancer cells. Our in-house mouse strains include CD1-Foxn1nu and SCID/beige, while other strains are available upon request.
Tumor treatment procedures encompass various routes of drug administration, including invasive methods (IV, IP, SC, and PO), noninvasive methods (via food), and photodynamic therapy (PDT) using a diverse range of irradiation wavelengths.
The deliverable for these studies consists of a comprehensive report that includes the study design, experimental data and interpretation, preliminary statistics, requested tissue samples, and terminal blood samples.
Deliverable: A detailed study report including the description of the study design, experimental data and interpretation, preliminary statistics, requested tissue samples, and terminal blood.
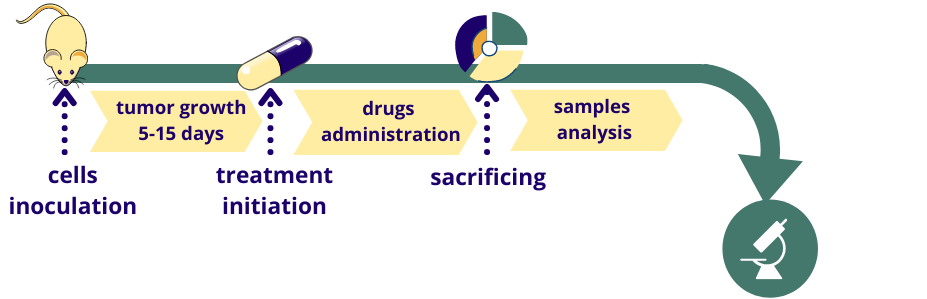
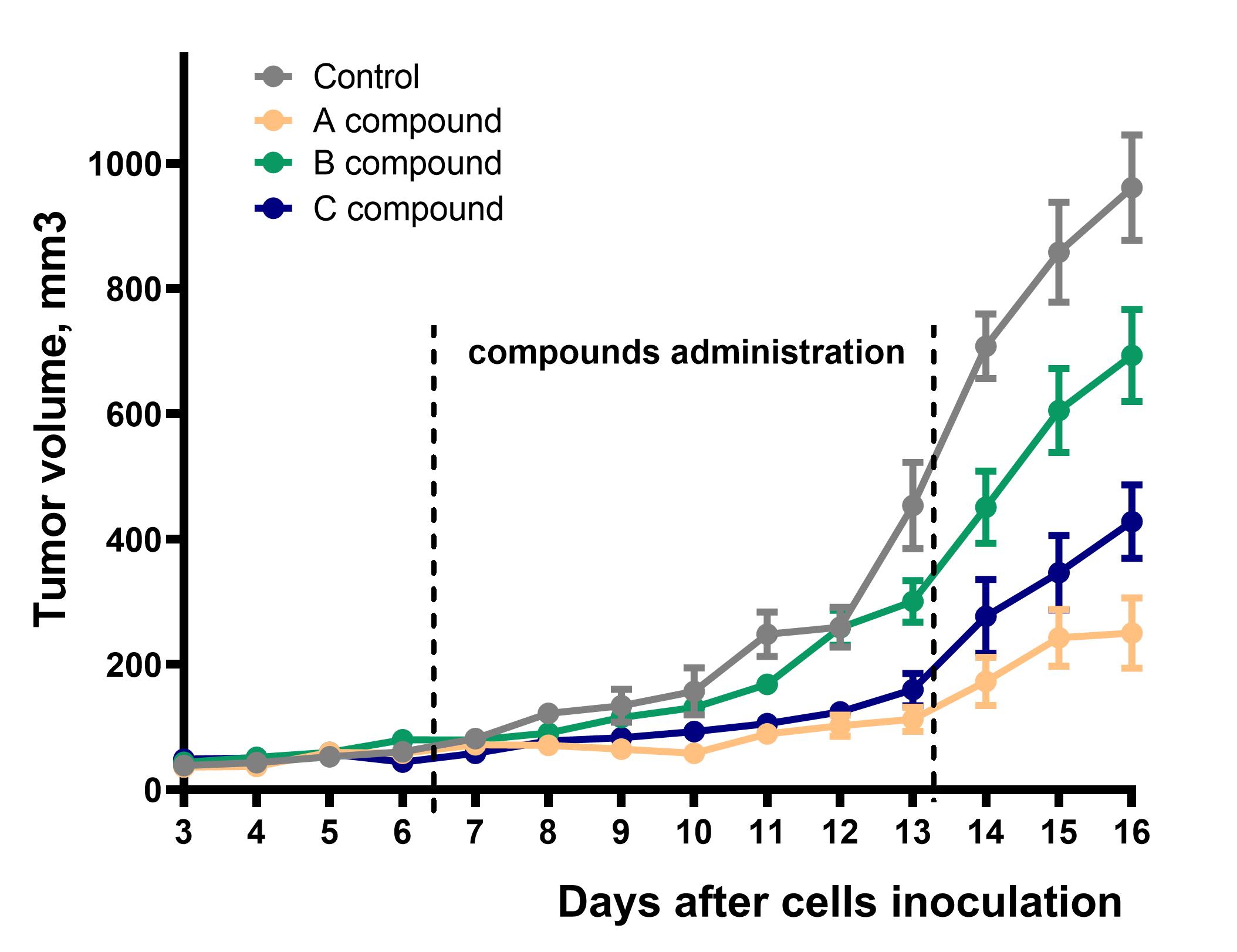
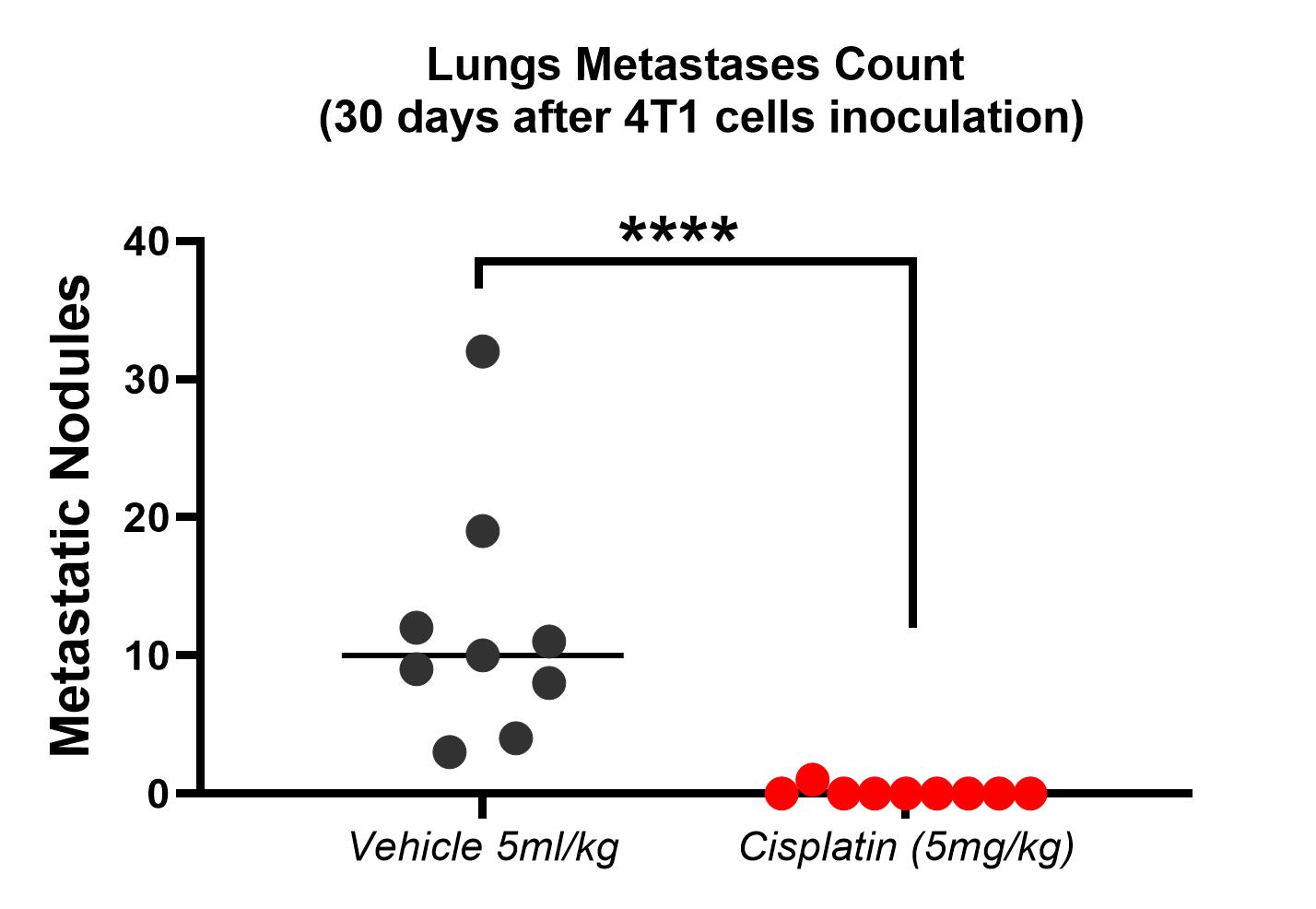
Service details: A standard study design includes 7 days of acclimatization, 5-30 days of tumor growth (depending on cancer cell line type), a study-specific treatment period, and post-treatment observation. A study design includes a minimum of 2 groups of mice (tumor-bearing vehicle-treated and tumor-bearing test compound-treated group). Drug administration can be performed via invasive (IV, IP, SC, and IM routes) or noninvasive (PO, IN, or diet incorporated) routes. Other specific types of treatment are also possible, for example, photodynamic therapy (PDT) using a wide range of irradiation wavelengths. Typically, animals are observed for clinical signs during the treatment and post-treatment periods. The tumor growth dynamic is carefully monitored. A gross necropsy is performed at the endpoint, and metastases are counted. Specific tests, such as hematological, urinary, and blood clinical chemistry analysis, food intake, histopathology, etc., combined with specific endpoints, are available on request.
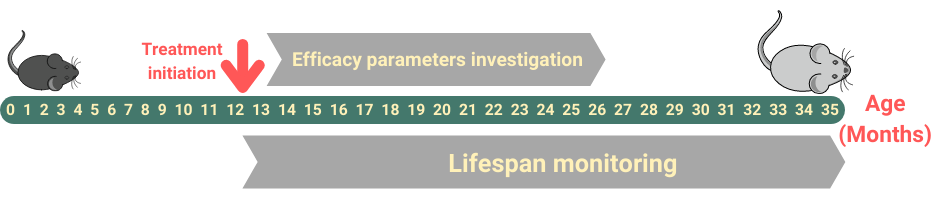
Aging is a risk factor for many age-related diseases, such as type 2 diabetes, atherosclerosis, cardiovascular pathology, cancer, retinopathy, sarcopenia, etc.With the increase in human life expectancy, there is a growing interest in the development of drugs that can delay the onset and progression of aging and age-related diseases. Anti-aging drug testing is carried out on murine models of natural aging, such as C57Bl/6J, Balb/c, or CD-1 strains.
Service details: A standard study design includes:
- At least two groups of male and/or female mice (vehicle-dosed and treated with tested compound); the “young” control group(s) on the customer’s request.
- Animals were maintained in good conventional conditions, 12-hour light/dark cycles, and free access to standardized mouse diets and acidified boiled tap water. If necessary, a modified diet is available.
- Treatment begins at the age of 2-3, 5-6, 10-12, 16-18, and 22-24 months (at the customer’s request).
- The customer offers doses and schemes of treatment.
- Animals are maintained until natural death or specified endpoint. Standard anti-aging effects are monitored by routine tests, such as mortality assessment (daily), body weight and physical condition (weekly), physical activity (quarterlyor at customized time points), and gross necropsy (post-mortem).
- Specific measurements and tests, such as hematological and clinical chemistry parameters, in combination with physiological and behavioral tests and more definitive endpoints, are available for an additional cost.
- Treatment procedures include invasive (by IV, IP, SC, and PO routes), and non-invasive (food-incorporated) methods of drug administration.
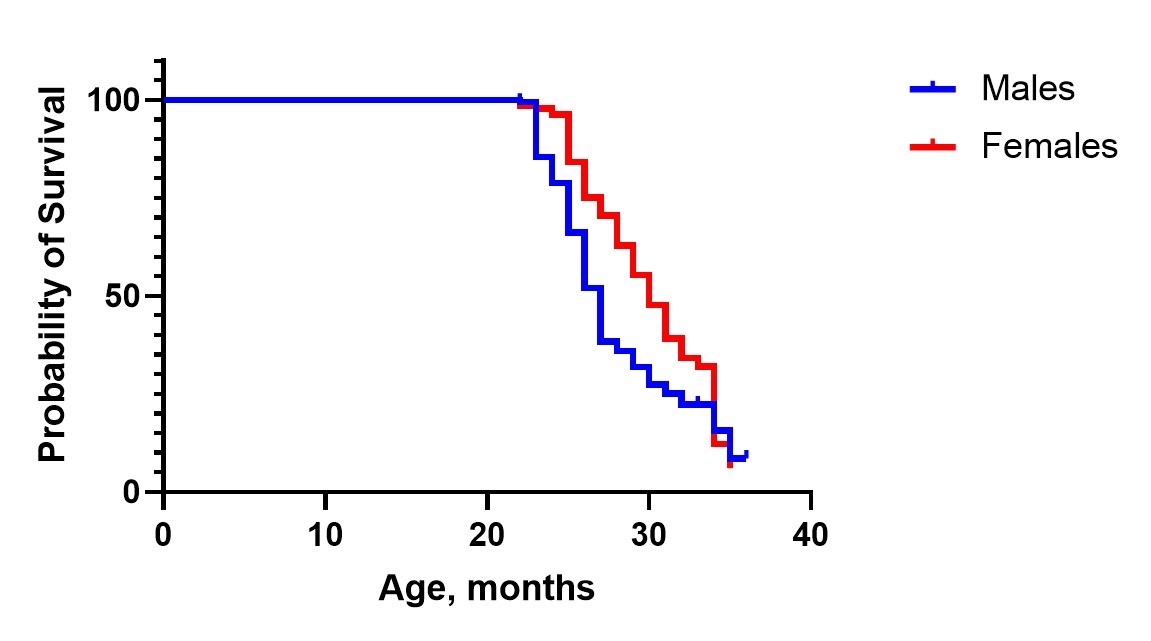
A typical survival curve of C57BL/6J female mice treated with metformin (M), aspirin (A), and both compounds (AM):
Animal efficacy models became an important step in determining the fate of new drug candidates in a preclinical drug discovery pipeline. Among all, mouse efficacy models are the most widely used in biomedical research and preclinical drug evaluation.
At Bienta, we developed several efficacy models for your drug efficacy studies
During the drug discovery process, preclinical/nonclinical toxicology studies are conducted to reveal a candidate molecule’s earliest potential toxic effects as it progresses from hit to lead and ultimately to a drug candidate, reducing the number of animals involved at later stages of drug discovery. Our skilled research team tailors the toxicity study protocol to meet the unique requirements of our clients. Bienta adheres to the OECD Guidelines for the Testing of Chemicals and FELASA recommendations for working with animals, and our Institutional Animal Care and Use Committee (IACUC) approves our protocols.
Our toxicology services:
Administration routes:
- PO, IV, IP, SC, IM, IN, with food.
We perform:
- Observation for mortality, signs of toxicity, and behavioral changes at all required time points after administration;
- Weight measurement;
- Food and water intake measurements;
- Gross necropsy with macroscopic inspection;
- Clinical Chemistry panel: Alkaline Phosphatase, Alanine Aminotransferase, Aspartate Aminotransferase, γ-Glutamyltransferase, Urea, Cholesterol, Creatinine, Creatine Kinase, Total Protein, Triglycerides, Lactate dehydrogenase, Total and direct bilirubin, High-density lipoproteins, and other custom parameters;
- Hematological panel: Leukocytes (MID, Lymphocytes, Granulocytes), Erythrocytes, Platelets, Hemoglobin, Hematocrit, etc (17 parameters).
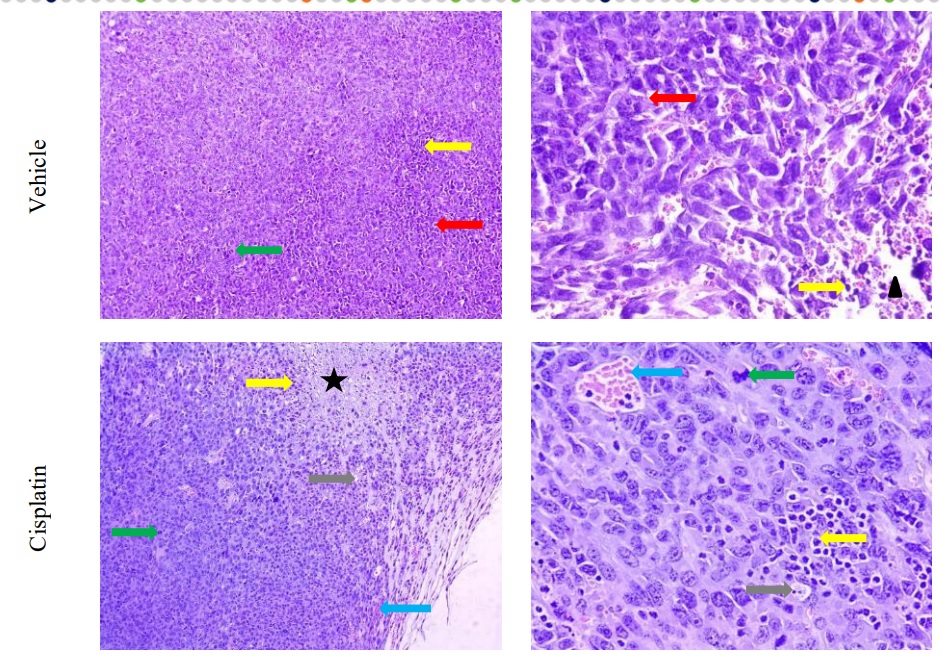
- Histopathological assessment: all the tissues included xenografted tumors (H&E staining, immunohistochemistry, other stainings on custom request both for FFPE and cryo-sections), morphometry on the custom request. Microphotographs are provided.
We provide all requested biological samples from experimental animals, such as plasma samples, flash-frozen tissues, paraffin- or cryogel-embedded tissues for histology, etc.
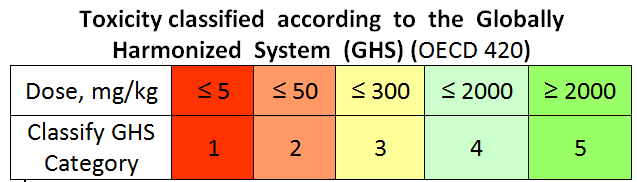
Toxicity class study
Toxicity class study is conducted to determine the class of toxicity of a substance. The results of the study help to narrow the range to search for a therapeutic dose. Fixed dose 5, 50, 300, and 2000 mg/kg are used in this study. The study requires 15 animals and 15 working days.
Dose range finding (DRF)
The dose range-finding (DRF) is an initial part of the toxicity study aimed to find the dose that will produce tolerable levels of adverse toxic effects of tested compounds. We investigate the adverse effects of acute doses administration and determine the different tolerable levels of doses – usually maximum tolerated dose (MTD), the no-observed-adverse-effect level (NOAEL) – depending on the aim of the study. Blood microsampling procedures are available to reduce the animal number. A customized DRF design is also available.
Adverse effects investigation:
- Observations for clinical signs and mortality;
- Gross necropsy with macroscopic inspection;
- Customized parameters by request (blood count, clinical chemistry, metabolic values, tissue histopathology, behavioral tests, etc.)
Deliverable: A detailed study report including the description of the study design, experimental data, and interpretation, preliminary statistics, and requested tissue samples. Raw experimental data are available upon request.
Sample Submission. Dry compound or compound in a pre-made animal dosing formulation. The amount depends on the dosing levels. For example, 1 mg of a compound will be enough for 10 mg/kg dosing.
Toxicokinetics in Rodents
The main goal of toxicokinetic studies is to establish a correlation between the compound concentration or dose and potential adverse effects and to aid in understanding the mechanisms of toxicity. Repeated dose toxicity studies in animals are performed for this purpose. Evaluation of various pharmacodynamic (adverse effects) and pharmacokinetics (absorption, biodistribution/accumulation, metabolism, excretion) parameters is usually performed after the first and last injection of the compound or drug candidate. Compound concentration in plasma is measured using the HPLC-MS/MS method. At Bienta, toxicokinetics studies are carried out in accordance with OECD 417 guidelines.
Typically, DRF is the first stage of a toxicokinetics study.
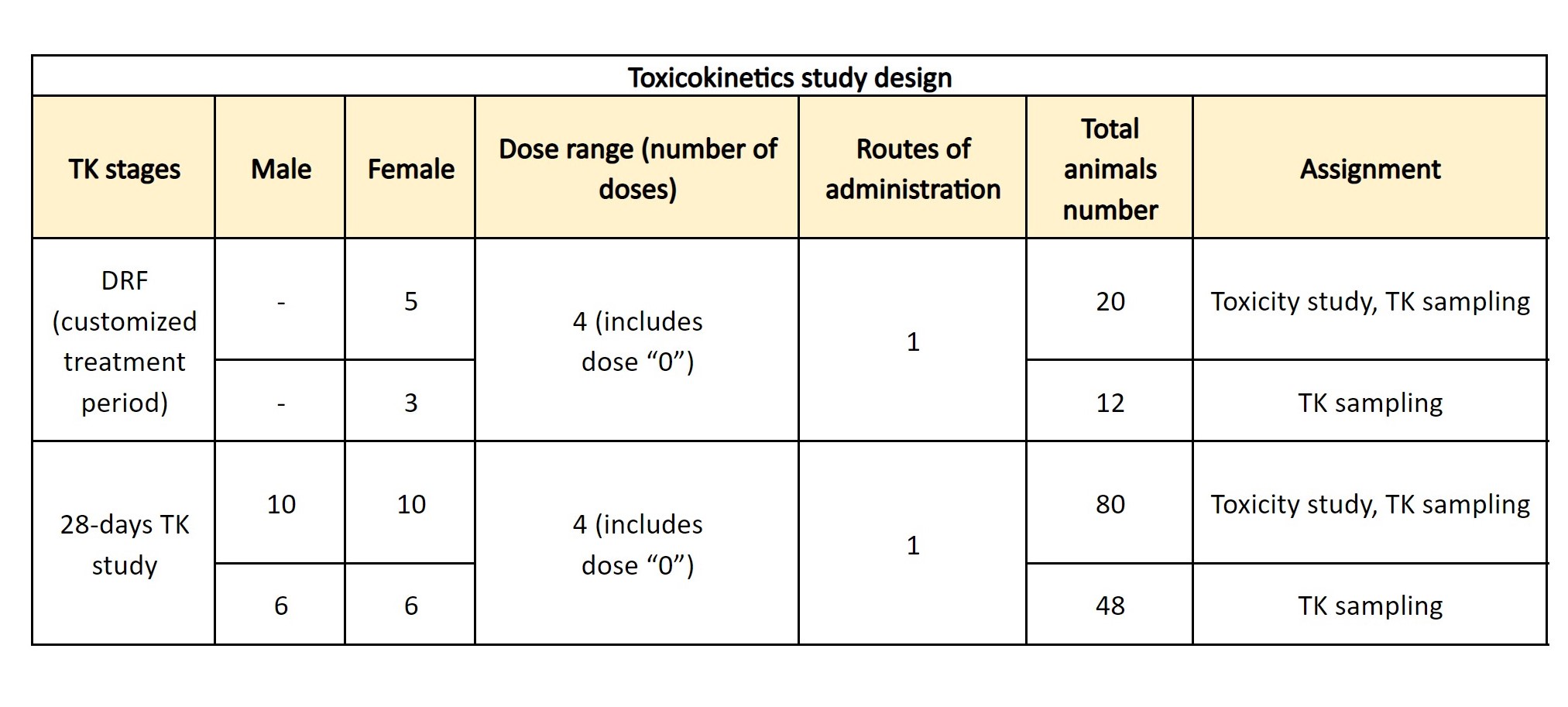
Repeated dose toxicity study
Repeated dose toxicity study is usually conducted with the aim to determine the specific dose (MTD, or NOAEL) after repeated dosage. Treatment design and duration both depend on a therapeutic treatment schedule. The study design includes 1-3 tested doses per route in animals of both sexes, 6-10 animals per group. On average, 30 animals and 30 working days are required for this study. A customized design is also available.
Pathology-specific side effects investigation:
- observations of clinical signs of toxicity
- hematology
- clinical chemistry
- behavior
- metabolic values
- customized parameters.
Deliverable: A detailed study report including a full description of study design, analytical method development, calculations of all PK parameters, and PK graphs. Raw experimental data are available upon request.
Sample Submission: Dry compound or compound in a pre-made animal dosing formulation. Amounts depend on the dosing levels. For acute toxicology study in the group of 30 mice at 10 mg/kg, approximately 10 mg of a compound is required.
Acute toxicity study
Acute toxicity studies are aimed to evaluate adverse effects after the administration of a single dose or multiple doses of a test substance given during a period not exceeding 24 hours. Acute toxicity studies are designed to determine:
- the dose that will produce either mortality or serious toxicological effects;
- time course of drug-induced clinical observations;
- the doses that should be used in subsequent studies;
- what effects the compound has on morphology, clinical chemistry, or other parameters;
- the possible target organ(s) of toxicity;
- the estimation of safe acute doses for humans.
Acute toxicity testing of potential new pharmaceutical products is traditionally conducted by at least two routes of administration: the intended clinical and a parenteral (IV or IP) route.
Service details. In the standard design, acute toxicity information is obtained from appropriately conducted dose-ranging studies that define an MTD (please, see appropriate service for details), followed by the next main phase for a more detailed study of toxic effects. In typical design in the main phase, we dose one-three groups of animals with 1-3 selected doses of the test article. The number of animals in the main phase is variable, but in general, it includes 5 rodents per sex per group. A vehicle dosing group is also included. Animals are observed for mortality and signs of gross toxicity at 30 min, 2, 4, and 6 hours after the administration and thereafter daily for a period of up to 14 consecutive days. A gross necropsy is performed on all animals at the terminal sacrifice. Specific tests, such as hematological, urinary, and blood clinical chemistry analysis, food intake, histopathology, etc., in combination with more definitive toxic or gross pathology endpoints, are available on request. The inclusion of specialized endpoints based on the pharmacology of the test article is also available.
Deliverable: A detailed study report including a full description of the study design and all experimental data. Organs and tissue samples or histological slices are also available on demand.
Sample Submission: Dry compound or compound in a pre-made animal dosing formulation. Amounts depend on the dosing levels and the design of the study.
Subacute/subchronic (repeated-dose) toxicity (MTD/NOAEL)
Subacute systemic toxicity is defined as adverse effects occurring after multiple or continuous exposure between 24 h and 28 days. Subchronic systemic toxicity is defined as adverse effects occurring after the repeated or continuous administration of a test article for up to 90 days or not exceeding 10% of the animal’s lifespan.
Service details: In the standard design, one-three groups of animals of both sexes are dosed with 1-3 pre-selected doses of a test article. Each compound-treated group as well as vehicle dosing group contains 5 animals of each sex. Animals are observed daily for signs of toxicity, body weights are recorded weekly, and food intake can be measured weekly. At the end of the experimental period, blood samples are collected for hematology and clinical chemistry analysis, and a gross necropsy is performed at the endpoint of the study. Histopathology is also available upon request.
Treatment design and duration of the research depend on a therapeutic treatment schedule. The standard study design includes 1-3 tested doses per route in animals of both sexes, 5 animals per group, and Vehicle dosed group. On average, 40 animals and 28-90 working days are required for the study. A customized design is available.
Chronic toxicity study
Chronic toxicity studies are performed for a period of 180 days to 1 year. During the study, the potential risks in relation to the anticipated dose and period of drug treatment, prospective targets of toxicity, and reversibility of clinical signs are observed.
Service details: Treatment design and duration both depend on a therapeutic treatment schedule. The standard study design includes 1-3 tested doses per route in animals of both sexes, 1 vehicle-dosed group, 20 animals per group. On average, 280 animals (including satellite groups) and 6-12 months are required for this study. Animals are observed daily for signs of toxicity, body weights are recorded weekly, and food intake can be measured weekly. Blood samples are collected for hematology and clinical chemistry analysis at selected intermediate time points and at the end of the test period. A gross necropsy is performed at all endpoints of the study. Histopathology is also available upon request. A customized design is available.
Immunotoxicity
Potential drug candidate development proceeds with the investigation of a variety of adverse effects including immune system failure. The service includes assessment of suppression or enhancement of the immune functions after treatment by testing the compound.
Service details: The service proposes various study designs to assess the suppression or enhancing the immune response in rodents, as a choice to develop hypersensitivity (allergic) reactions after exposures to the testing drug in guinea pigs. Standard study design in rodents includes investigation of hematological parameters, immune organ weights/cellularity (thymus, spleen, lymph nodes, bone marrow), serum immunoglobulins level. Specific immunological tests are available – antigen-specific plaque-forming cells testing, RBTL in vitro, immunophenotyping of leukocyte populations in the blood or lymphoid tissues, and other customized methods.
Acute Dermal Toxicity
Acute dermal toxicity study is used to identify potential side effects following skin application of a test substance. The test is performed after a single dose or multiple doses of the substance which have been applied to the skin over a 24-hour period.
Service details: In a typical design, two-five groups of female rats are dosed with 1 to 4 pre-selected doses of a test article. The test compounds are applied over the exposed area of dorsal/flank skin (~ 10 % of the total body surface area). Each compound-treated group as well as the vehicle dosing group contains 3-5 animals. Animals are observed for mortality and clinical signs of toxicity at 30 min, 2, 4, and 6 hours after the administration and thereafter daily for a period of 14 consecutive days. Individual bodу weights of animals are determined on the day of the compound administration and every two days thereafter. Gross necropsy is performed for all animals at the endpoint of the study. Specific tests, such as hematological, urine and blood clinical chemistry analysis, water and food intake, histopathology, etc. are available on request. Implementation of specialized endpoints based on the pharmacology of the test article is also available.
Infusion toxicology
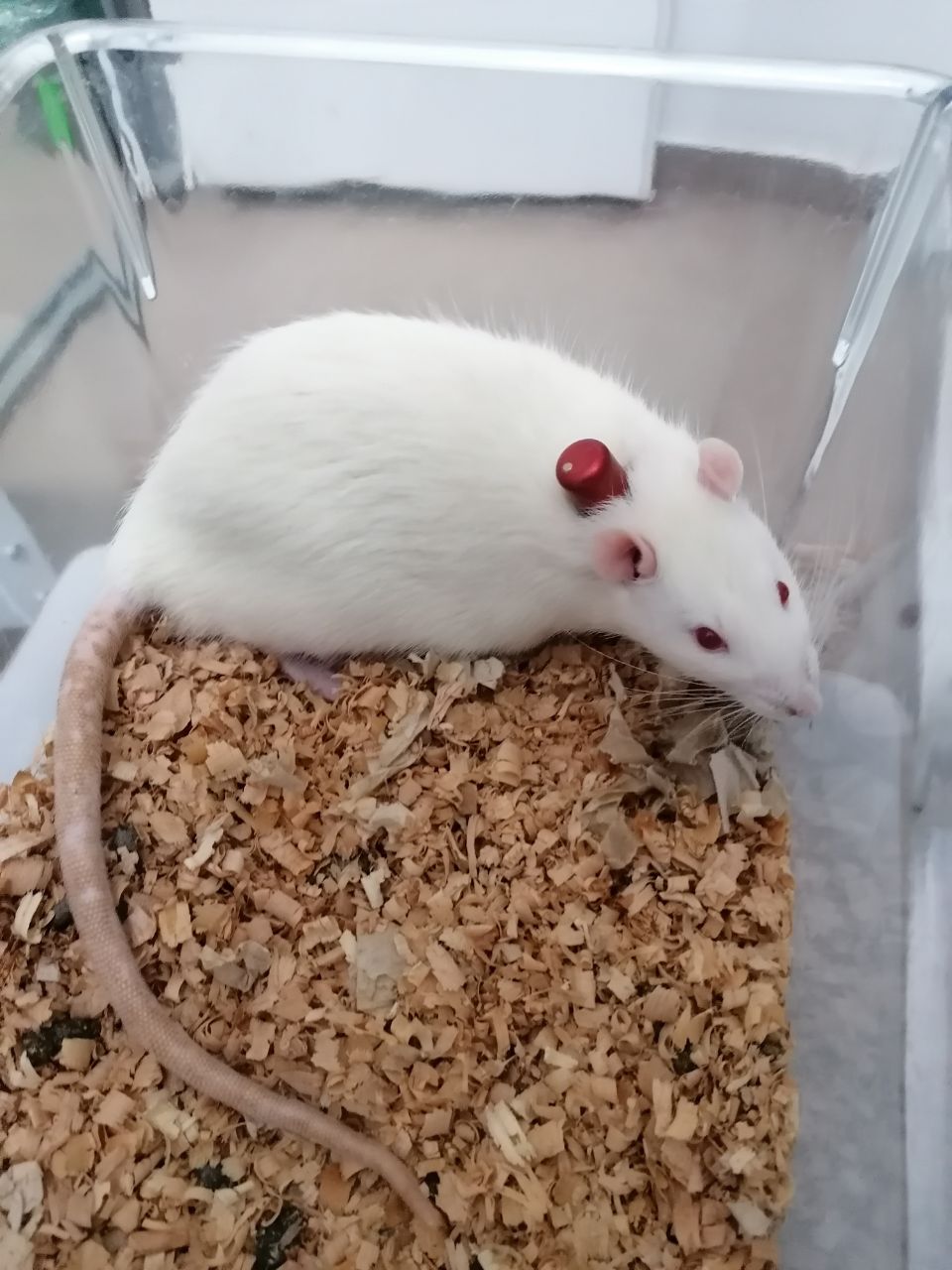
Infusion toxicology in preclinical studies are meant to asses the safety of therapeutic candidates intended for continuous administration in clinics. Slow infusions are possible through vascular access button. Bienta’s specialists possess necessary experience for the installation of the access buttons. Shortly, after the procedure animals are recovered and ready for a study.
Background and Service Details:
Enzymatic biotransformation of drugs in living systems strongly affect their biological activity, which sometimes results in metabolite with decreased bioavailability and enhanced toxicity. The knowledge of specific sites of metabolic transformations is useful for guiding synthetic optimization of the lead compounds or drug candidates to overcome the stability and toxicity issues.
To elucidate the main routes of hepatic metabolism, incubation of test compounds is carried out with liver microsomes (mouse, rat or human), that provide a wide range of the metabolizing enzymes. Additionally, S9 fractions from mouse, rat or and human liver can be used for a broader coverage of possible biotransformations (both phases I and II of metabolism). Some compounds are unstable in blood plasma, therefore plasma stability test can be included in the service. A typical protocol includes incubation of the test compound with microsomes or S9 in the presence of co-factors, sampling at 2 time-points (0 and 60 minutes), reaction quenching and centrifugation. The analysis of the incubation sample and comparison to the control (quenched at 0 time) is performed using liquid chromatography/tandem mass spectrometry.
Our approach to drug metabolite identification integrates multiple reaction monitoring of predicted metabolites with information dependent acquisition (MRM-IDA) using hybrid triple quadrupole linear ion trap mass spectrometer API5000 QTRAP (AB Sciex). The multiple reaction monitoring with information dependent acquisition MRM-IDA methods are created using LightSight 2.3 software (AB Sciex) comprising a comprehensive database of all classical metabolic biotransformations, both phases I and II of metabolism. The methods comprise the survey MRM-IDA scans for parent compound and metabolites linked to information dependent enhanced product ion (EPI) scan. The fragmentation pathways of parent compound and metabolites are elucidated by analysis of MS/MS product ion spectra obtained from enhanced product ion (EPI) scans. The identification of metabolites and assignment of MS/MS product ions with specific fragments of molecule is performed using ACD/Labs MS Fragmenter software.
Deliverable:
Data include ion chromatograms of parent compound and metabolites, the table containing metabolites references and where possible molecular formulae, types of biotransformations, masses, m/z, mass differences from the parent, and retention times. The structure identification data comprise MS/MS spectra and details of the product ion fragments for parent compound and metabolites, as well as structural assignment based on the key fragments observed. Finally, we provide a detailed report containing proposed metabolites structures and expected metabolic pathways.
Sample Submission:
A minimal accurately weighable quantity of dry compound (~1 mg or 2 µmol) or 50 µL of 10-20 mM stock DMSO solution is required for this assay. For multiple assays, smaller amount of compound per assay may be sufficient, which should be discussed for each particular project. Molecular structures are necessary for metabolite profiling studies.
Pharmacokinetic (PK) studies are part of our ADMET panel. The study design can significantly vary depending on the goals and parameters of the tested compounds. We offer PK in widely used mouse inbred (C57BL/6, BALB/c), outbred (CD1, NMRI) strains, and Wistar, or Sprague Dawley rats. All study protocols are reviewed by Bienta’s Institutional Animal Care and Use Committee (BACUC).
A typical PK study in mice involves two drug delivery routes (e.g. PO and IV), 6 time points for each route (for example 5, 15, 30, 60, 120, 240 min for IV and 15, 30, 60, 120, 240 and 360 min for PO) and 4 animals per each time point group/route, plus the common control plasma of Vehicle dosed group, 50 animals in total. We will prepare blood plasma samples and measure compound concentrations by LC-MS/MS using AB Sciex API4000/3000 mass spectrometers and Shimadzu (Prominence, VP) HPLC systems.
We offer:
- Measurements of various pharmacokinetic and pharmacodynamics parameters;
- Compound absorption/elimination dynamics following different routes of administration;
- Biodistribution (brain, liver, kidney, lung, spleen, heart, liquor, etc.);
- Metabolism/biotransformation – metabolite ID services;
- Compound excretion dynamics (urine/feces, bile);
- End-point or serial sampling;
- Single compound or cassette pharmacokinetics for a mix of 2-3-4-5 compounds;
- Wide range of time points;
- Native or perfused tissue sampling;
- Development of optimized drug delivery formulations to improve the bioavailability (Formulation Screen)
Deliverable: A detailed study report including a complete description of the study design, analytical method, measured test article concentrations, all common PK parameters, and PK graphs. Raw experimental data are available upon request.
Sample Submission. Dry compound or compound in a pre-made animal dosing formulation. Amounts depend on the dosing levels. For a PK study in mice at 10 mg/kg PO and 10 mg/kg IV, about 16 mg of the compound is required. We do not need to know the structures of the molecules for ADME testing. However, brutto formulas have to be provided for all studies involving MS detection.
BALB/cAnN

BALB/cAnN is an inbred mice strain. Haplotype: H2 d. High breading performance. Balb/c mice are widely used for general experimental studies in toxicology, pharmacology, teratology, and parasitology. Commonly applied for more specific purposes: obtaining hybridomas and monoclonal antibodies, infectious diseases studies, hippocampus-related diseases, oncology (breast cancer), and behavioral research. Due to the low level of spontaneous tumors, the strain is often used in long-term experiments, being a popular model in gene-engineering experiments. Being relatively resistant to diet-induced atherosclerosis BALB/c mice are a useful model for cardiovascular research. Balb/c mice are noted for a high level of anxiety. Sensitive to radiation. In later life might develop some types of cancers. This strain is sensitive to Listeriosis, all types of Leishmani, and some types of Trypanosomes. Female mice have low productivity: only 47% of couplings are effective.
C57Bl/6
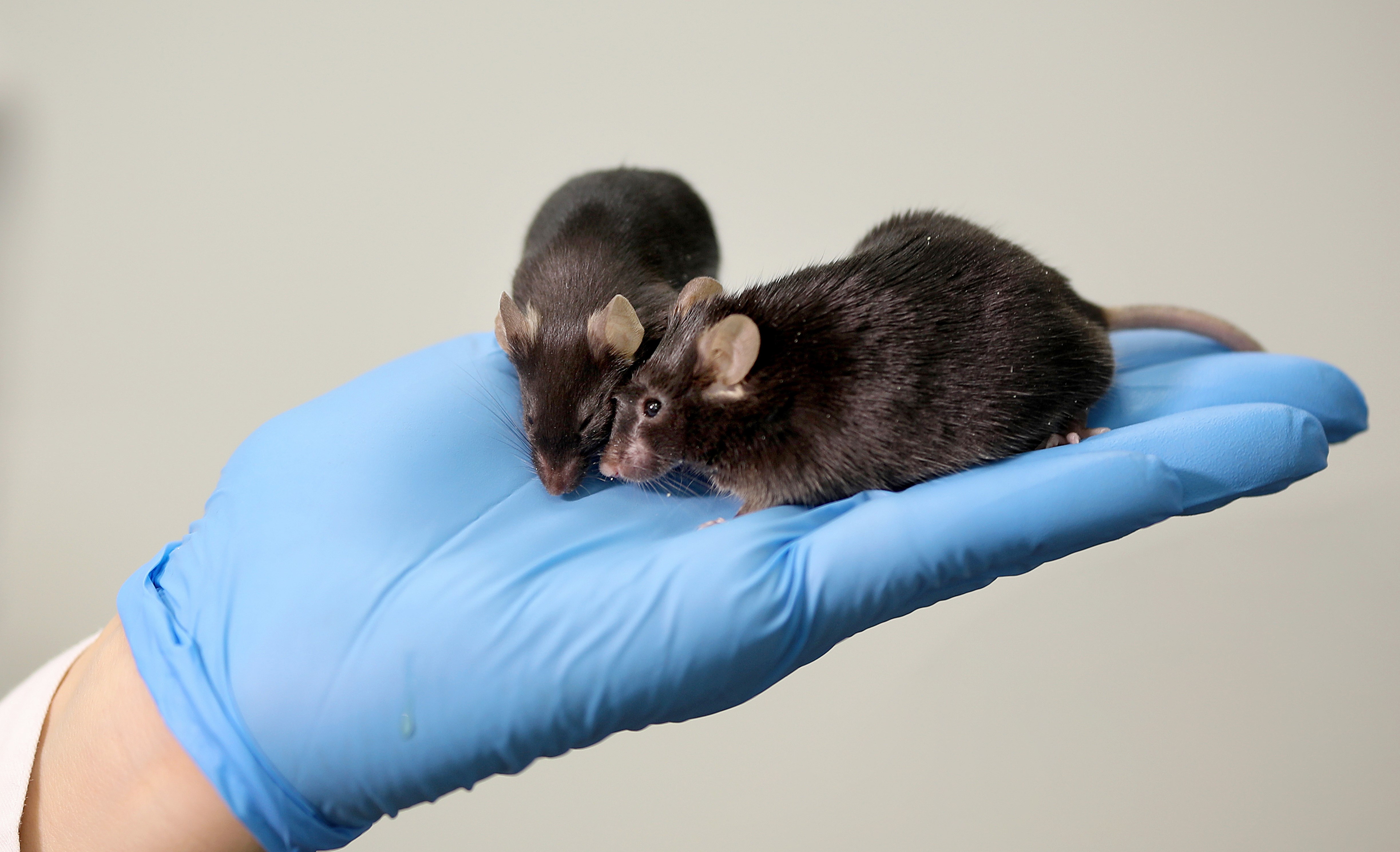
C57Bl/6 is an inbred mice strain. Haplotype: H2 b. The coat’s color is black. This strain is often used as a model for diabetes, obesity, and atherosclerosis. Two substrains (6J and 6N) differ in diet-induced obesity development: 6J is more susceptible. Due to their physical activity, they are suitable for behavioral studies. Also, they are commonly used as a knockout model. Mice C57Bl/6 do not develop breast tumors. However, particular features of these mice consist of partial abnormal development with defects of the skull, spine, limbs, and eyes/vision. The strain has high productivity: from 84% to 87% couplings are effective.
CD-1

CD-1 strain is an outbred mouse with white coat color. Developed from Swiss mice at the Anti-Cancer Center in Switzerland. The strain is widely used as a cost-effective model for preliminary studies in toxicology, oncology, pharmacology, immunology, and virology. Well suited for aging research. CD-1 mice strain is suitable for behavioral research and as a surgical model. The strain has perfect breeding qualities (up to 93%) and active/aggressive behavior. The typical litter size is 10-13 offspring.
C3H/HeOuJ
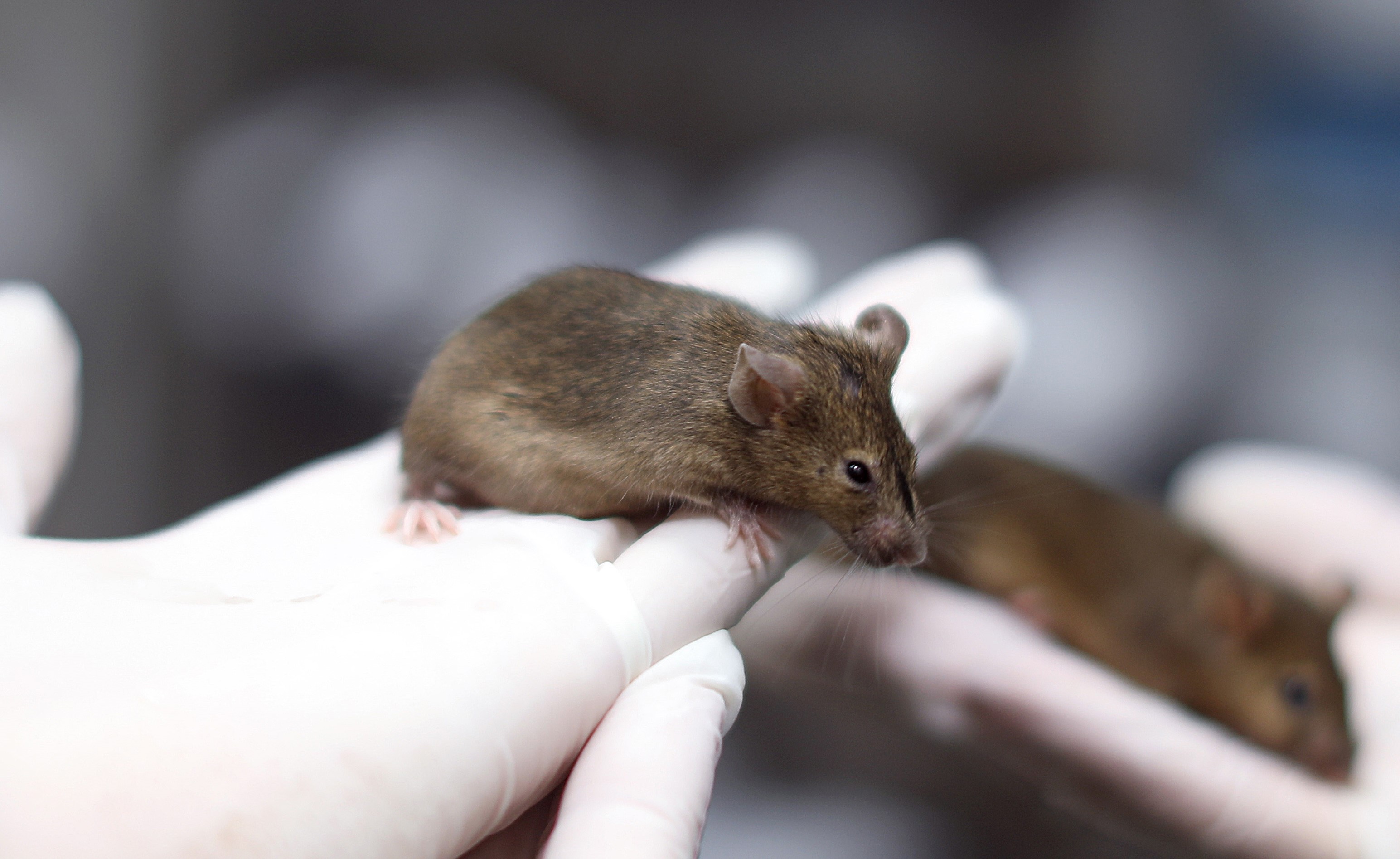
C3H/HeOuJ is an inbred mice with an agouti coat color. The strain was created by the mating of albino females with DBA males. C3H/HeOuJ mice are mainly used in immunology, parasitology, virology, oncology, and immunogenetics. Also, they are commonly used in sensorineural and cardiovascular studies. C3H/HeOuJ mice are sensitive to Gram-negative infection. Female mice are characterized by high levels of breast tumor development. Mating productivity is very high (up to 100%), however, the litter is smaller, compared to the other strains (2-5 offspring).
NMRI (Han)

NMRI is an outbred mice strain with white coat color. Obtained from inbred strain NIH/P1. The strain is commonly used as a model for toxicology, oncology, pharmacology, teratology, immunology, virology, and neurology. Most suitable as a behavioral and surgical model. NMRI mice have an age-related increase in spontaneous tumors and kidney diseases. Very high level of productivity: 86-92%, with a litter of 10-13 offspring.
CD1-Foxn1nu

CD1-Foxn1nu are commonly used for modeling different cancer types for biomedical research purposes. Homozygous CD1-Foxn1nu are athymic mice, and not able to produce T-cells, and have a partial defect in B cell development, resulting in an inhibited cell-mediated immunity. Due to this characteristic CD1-Foxn1nu mice are widely used for xenograft tumor implantations.
SCID/beige
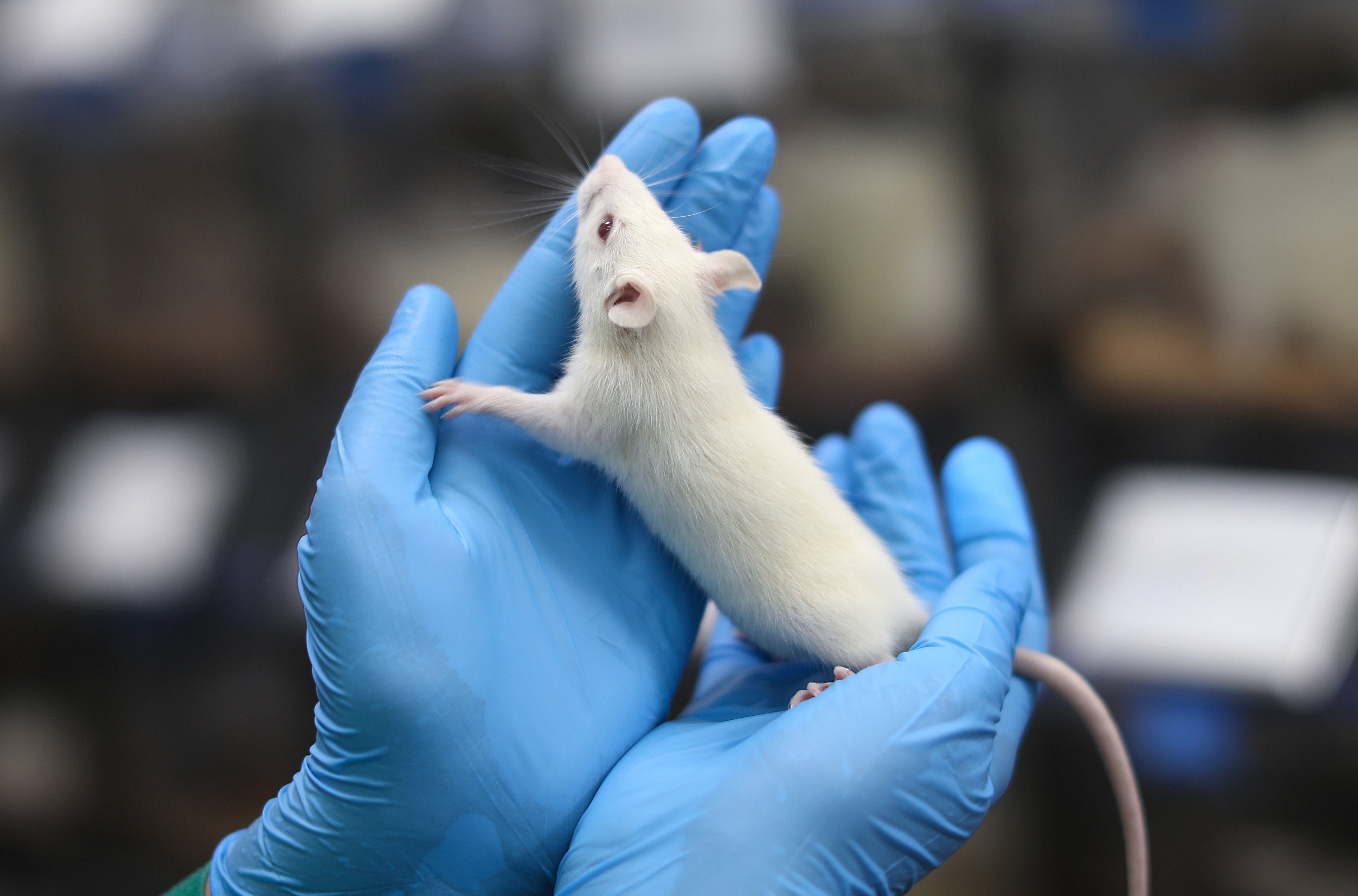
SCID/beige mice carry the Prkdcscid mutation which causes a lack of both T and B lymphocytes. Mice also have Lystbg-J mutation which results in cytotoxic T cell, macrophage, and NK cell function impairment. As a result, SCID/beige mice are sufficient models for the receipt of xenotransplants.
Bienta is equipped with the fully automated confocal microscopy imaging system IN Cell Analyzer 6500HS from GE Healthcare.
Options for High Content Imaging confocal microscopy:
- 384-well format as standard;
- 2-5 markers could be imaged simultaneously;
- adherent and suspension cell lines;
- wide variety of cancer cell lines, and primary rat and mice cells are available at Bienta;
- IN Carta analysis software enables quick and robust quantitative analysis of images acquired on IN Cell Analyzer systems.
Examples of possible applications of IN Cell Analyzer 6500HS:
- Cell morphology changes (epithelial-to-mesenchymal transduction, cell differentiation);
- Organelles studies (number, size, localization, structure);
- Subcellular changes (translocation, trafficking, expression of proteins, co-localization of molecules);
- Cell viability;
- 3D cultures;
- Co-cultures (cell-cell interactions including cancer-stroma);
- Cell migration and invasion (wound-healing assay, MMP activity);
- Imaging of tissue samples labeled with fluorescent dyes
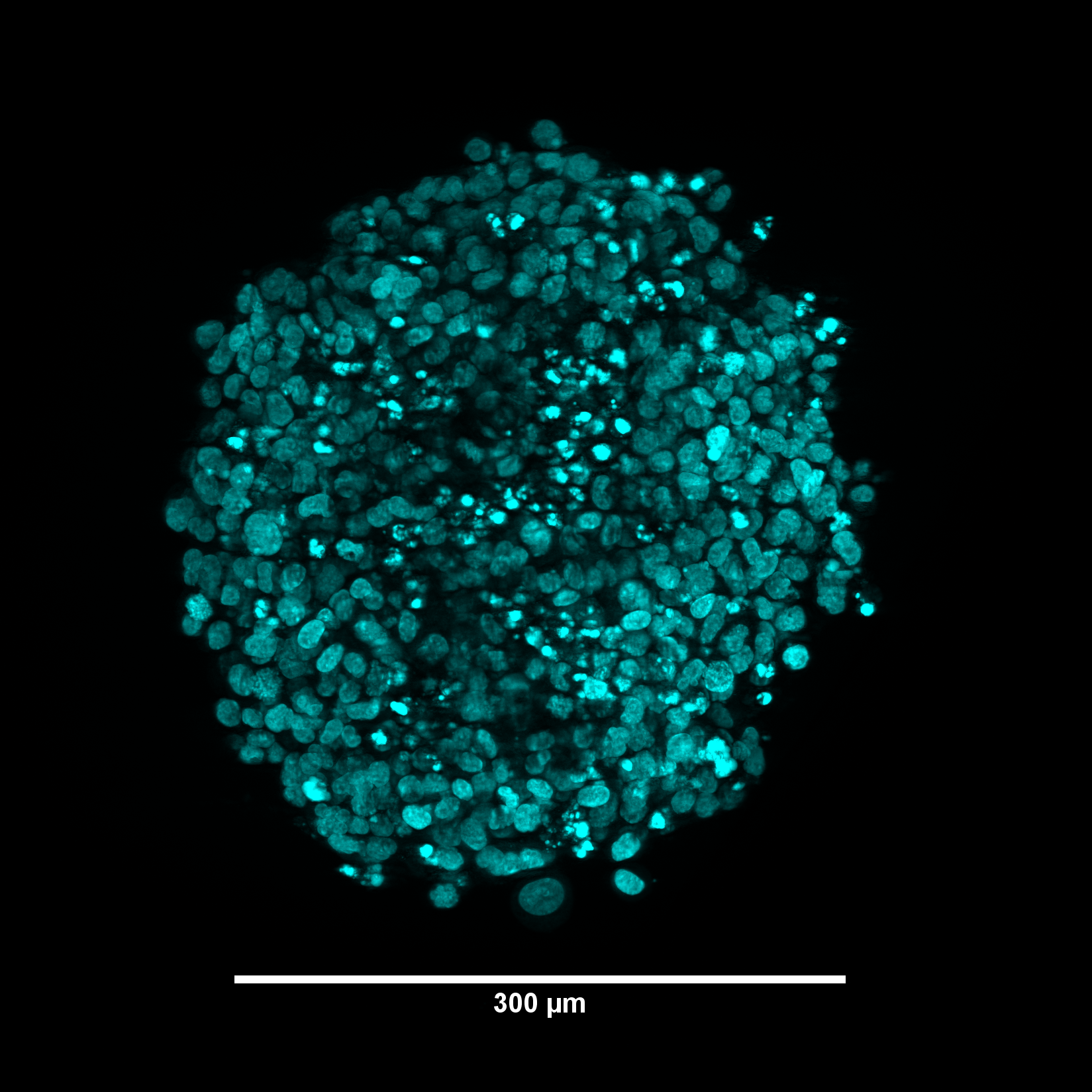
3D cancer cell culture. Max intensity projection image of the HeLa tumoroid stained with Hoechst 33342.
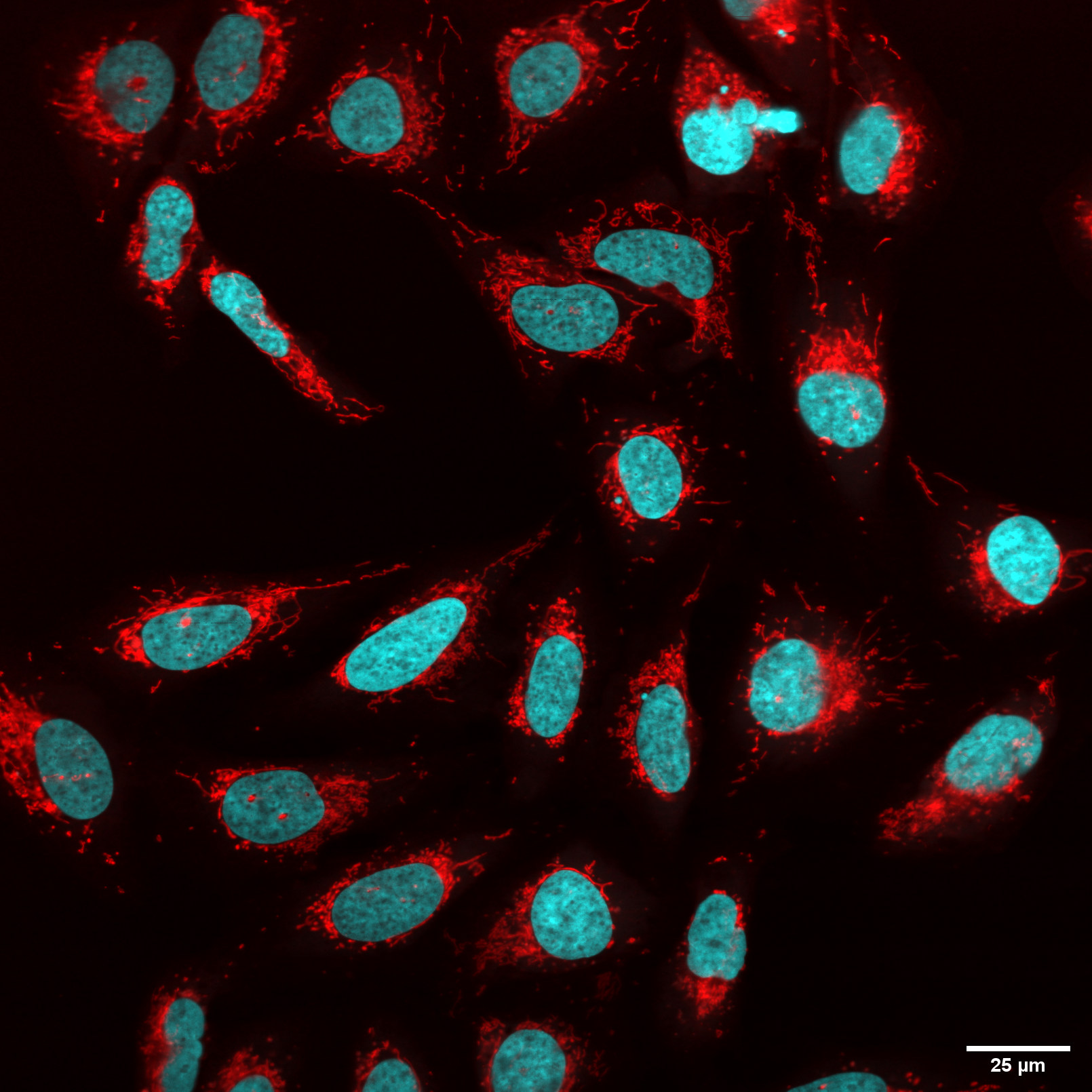
Detection of the mitochondrial membrane potential in U-2 OS cells. Red – mitochondria stained with MitoTracker Red CMXRos, cyan – nuclei stained with Hoechst 33342.
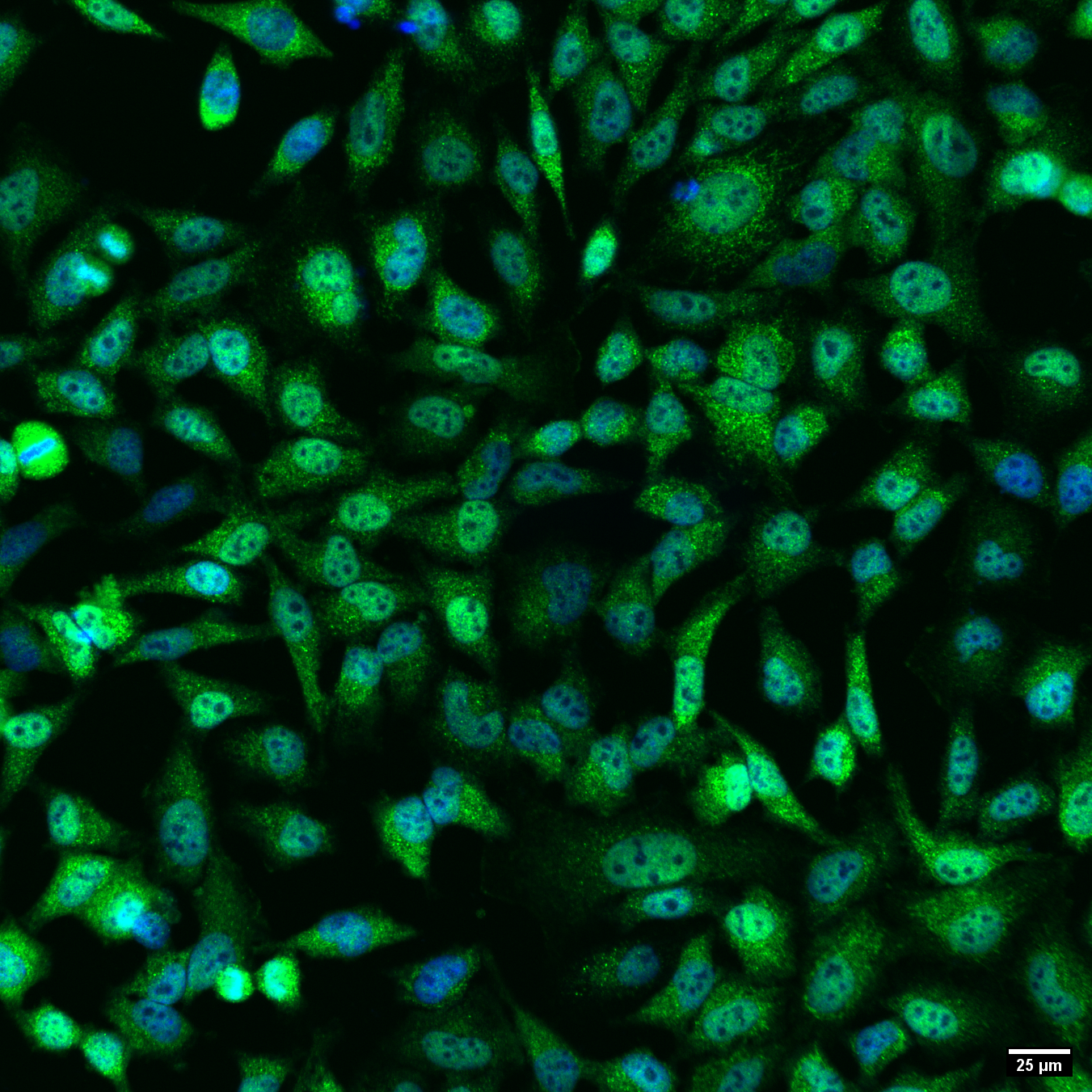
Nuclear transcription factor translocation assay. Confocal image of HeLa cells stained with antibodies against transcription factor of interest (green), and Hoechst 33342 (blue).
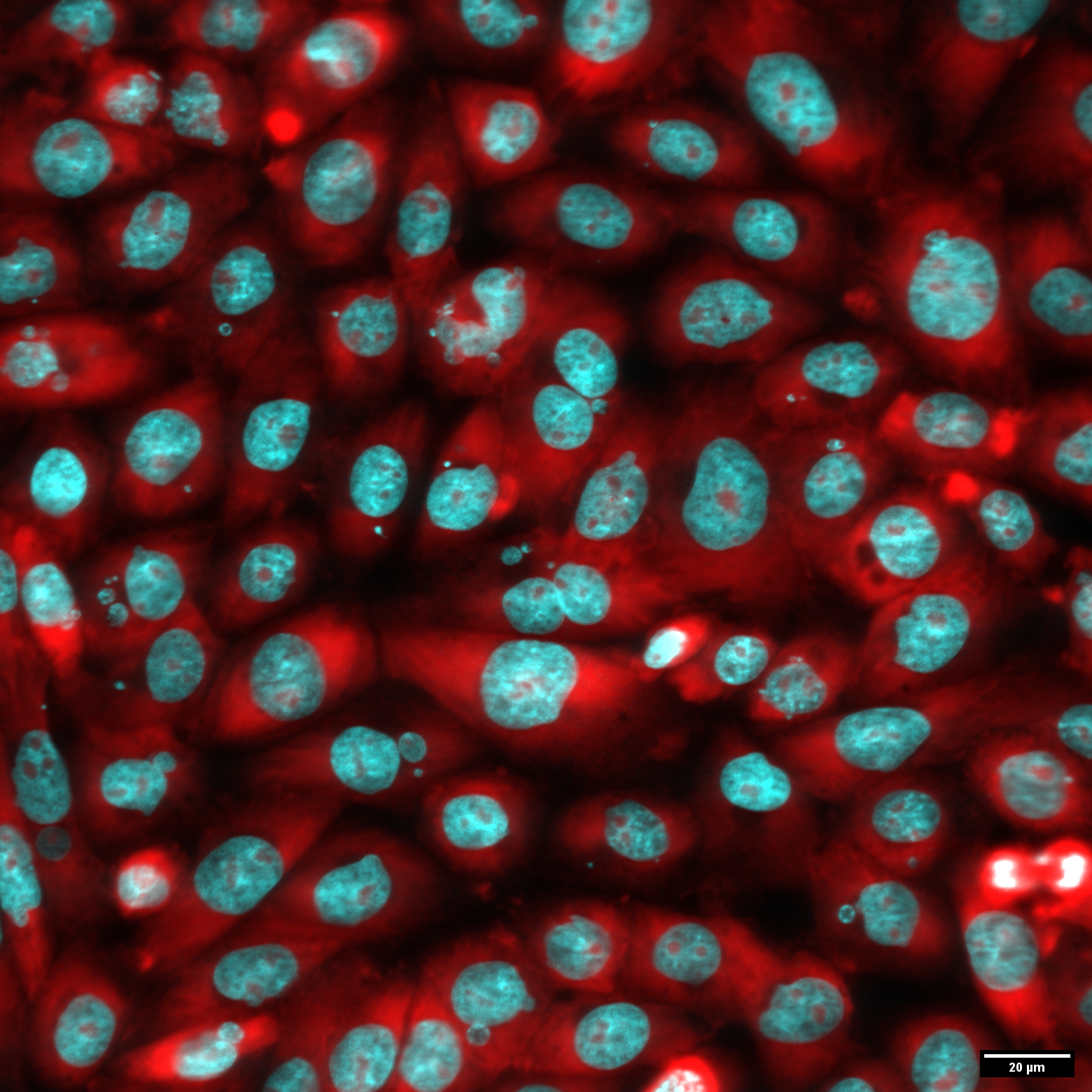
In vitro Micronucleus test. Confocal image of CHO-K1 cells treated with Mitomycin C. Cyan – nuclei and micronuclei of the cells were stained with Hoechst 33342, red – cytoplasm was labeled with Pyronin Y.
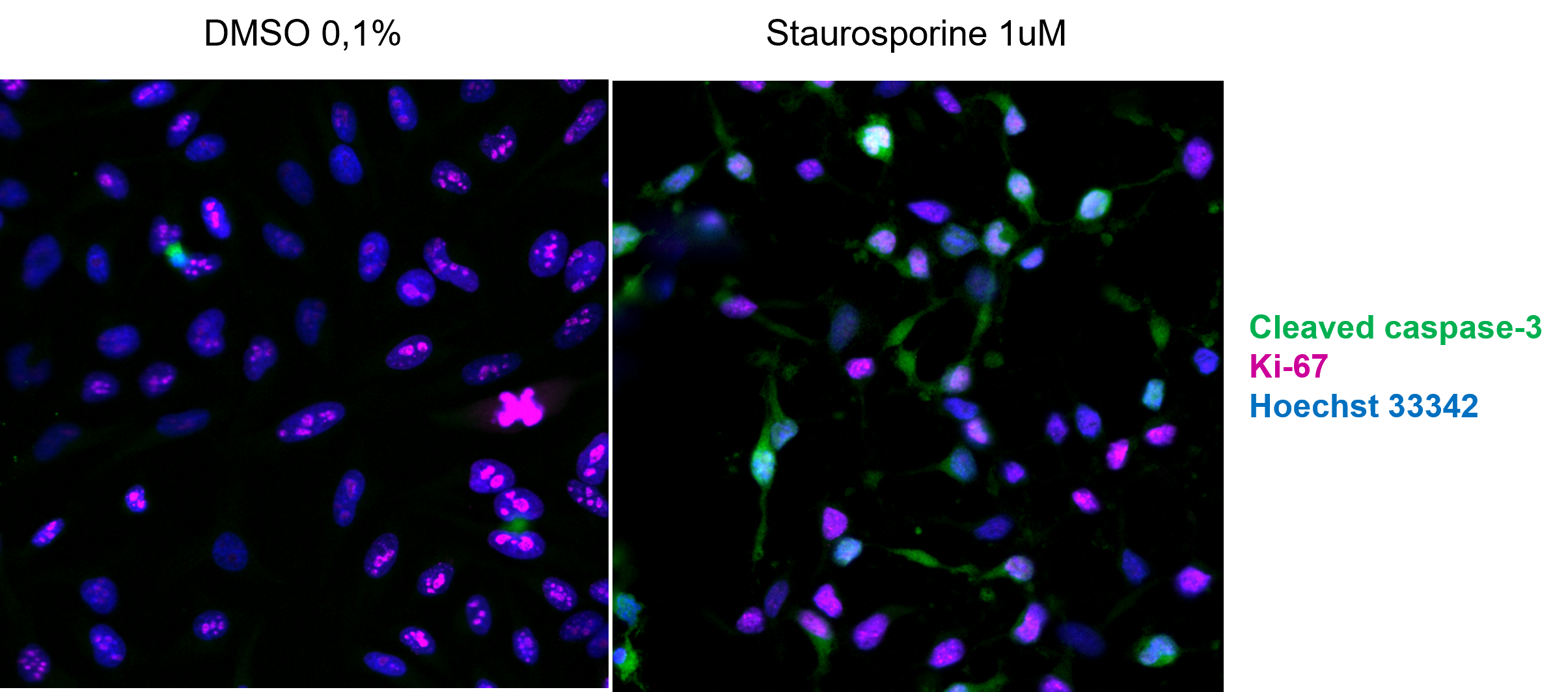
Apoptosis assay. Detection of the apoptosis using anti-cleaved caspase-3 antibodies (green) in HeLa cells treated with Staurosporine.
Surface plasmon resonance (SPR) is a sensitive biophysical method for the secondary screening of low molecular weight compounds and hit confirmation. Advantages of SPR methods include a low amount of target protein consumption, real time and label free detection of the affinity and kinetics of molecular interactions.

Fig.1 Experimental setup of surface plasmon resonance assay
Shortly, SPR experiments involve capturing the target protein on the chip surface and detecting its interaction with compounds in the aqueous solution. To provide our customers with the best quality results, we prefer to use chips with neutravidin/streptavidin coated surface and biotinylated AviTag proteins. In this matter, we ensure uniform protein orientation and equivalent compound access to the active site of the protein. On request, the biotinylated protein can be produced at Bienta
We can provide study of binding kinetics and affinity analysis in asensitive and highly efficient manner .
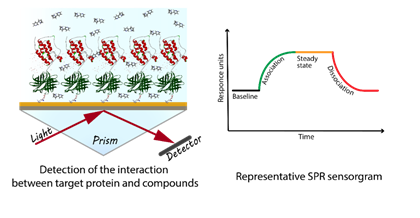
Fig.2 Principle of surface plasmon resonance assay
Our laboratory is equipped with state-of-the-art Biacore T200 instrument from GE Healthcare, which measures the small changes in the mass on the chip surface at the moment of interaction. Records about these changes are generated in sensorgrams that contain an information about association and dissociation of the compounds with the target protein.

SPR services for drug discovery:
- Experimental setup
- High-throughput screening:
- Single point 100 compounds/day
- Dose-response 10-30 compounds/day
- Data analysis
The Screening Group provides hit identification and hit-to-lead optimization contract services to the biotech and pharmaceutical industry. We are also opened to discuss shared-risk exploratory hit finding projects with the industry or academic partners, including the discovery and optimization of various assays and pharmacological tool compounds for new or poorly validated targets.
HTS Laboratory at Bienta is a well-equipped screening facility with a broad range of robotic liquid handling and signal readout technology capabilities. We have the capacity to run both biochemical and cell-based assays in 384-well plate format.
Assay read-out platforms:
- UV-VIS absorbance
- luminescence
- fluorescence intensity
- fluorescence polarization
- time-resolved fluorescence/energy transfer (FRET, TR-FRET, HTRF, DELFIA, LANCE)
- cellular calcium mobilization (FLIPR)
- ELISA
- AlphaScreen
- enzyme/reporter assays
- cell viability assays
- surface plasmon resonance
- cell confocal microscopic imaging
Our Protein Thermal Shift Screening Platform (or Differential Scanning Fluorimetry) includes ViiA 7 System (Applied Biosystems). Also we have two FluoDia T70 (Photal) readers.
Our strategy of “smart screening” relies on the extensive use of our chemoinformatics and molecular modelling resources and iterative access to Enamine’s on-site compound repository of close to 2,000,000 compounds after each round of experimental screening of in silico generated compound sets. Optimization of the obtained hits/leads can be facilitated by on-site access to Enamine’s high-throughput parallel chemistry lab. This unmatched combination of the powerful resources sets us apart from any competition.
Bioanalytical Laboratory at Bienta is established to provide common in vitro ADMET (absorption, distribution, metabolism, excretion and toxicity) tests as well as pharmacokinetics studies in small animals either separately or as a component of integrated drug discovery projects including screening and/or medicinal chemistry services by Enamine.
Current ADME-Tox Capabilities:
Physico-Chemical Properties
- Aqueous Solubility
- Shake flask solubility using Millipore or pION Solubility Filter Plates (UV or MS-MS detection)
- Laser nephelometry
- Chemical Stability Assay
- Log D/Log P
- Determining pKa
In vitro metabolism
- Metabolic stability in microsomes, hepatocytes and S9 from various sources (mouse, rat, human)
- CYP 450 inhibition assay (fluorogenic)
- CYP450 Time Dependent Inhibition (IC50 Shift Assay)
- LC-MS Based Cytochrome P450 Inhibition Assay
- Cytochrome CYP450 Reaction Phenotyping
- Plasma stability assay (human, rat, or mouse)
- Hepatic Microsomal Stability (human, rat, or mouse)
- Single Time Point Microsomal Stability Pre-Screen
- In Vitro Metabolite Profiling and Identification
In vitro permeability
- Membrane permeability
Protein binding studies
- Plasma Protein Binding Assay (Equilibrium Dialysis)
- Tissue Binding Assay (Equilibrium Dialysis)
- Microsomal Binding Assay (Equilibrium Dialysis)
In vitro toxicity studies
- Cytotoxicity
- various cell lines HepG2 (hepatocarcinoma), SK-N-DZ (neuroblastoma), MCF-7 (breast adenocarcinoma) and others
- Bacterial reverse mutation test (Ames test)
- Micronucleus test
- FluxOR™ hERG Assay
Animal studies
We are constantly expanding the list of standard analytical services offered. In addition, our experienced bioanalytical group can develop custom services according to the client’s specifications. Entrusting ADMET/PK testing to Bienta is a natural choice for the chemistry clients of Enamine since it translates into the inherent time- and cost savings to the customer.
We also work with any compounds shipped in from the outside, full confidentiality guaranteed.
In order to continue expanding our services in preclinical toxicology and animal efficacy models, in 2016, Bienta launched the new Animal Research Facility for in vivo studies in rodents. We claim that Bienta strictly follows internationally accepted guidelines for ethical animal care (Bienta/Enamine Animal Work Statement). All studies are approved by Bienta’s Institutional Animal Care and Use Committee (BACUC). Protocol extracts are available upon the customer’s request.
We offer:
- Pharmacokinetics Studies in Rodents
- Basic Formulation Screening for In Vivo Studies
- Toxicology Services
- Drug Efficacy Studies
- Aging Research
- Cancer
- Behavior
Animal species:
- Mice strains:
- Balb/cAnN,
- C57BL/6J,
- CD1(ICR),
- NMRI(Han),
- C3H/HeOuJ,
- CD1-Foxn1nu;
- Rats strains:
- Wistar,
- Sprague Dawley,
- SHR
- Other animals: (hamsters, guinea pigs, gerbils) are available on request.
Our Molecular Modeling Group provides cheminformatics, molecular modeling, virtual screening and computational biology services aiming to accelerate hit identification, lead generation and lead optimization stages of the drug discovery process. The main products of our Molecular Modeling group are the virtual and physical compound libraries (also called targeted libraries) for screening and diversity-oriented synthesis.
Rapid access to vast Enamine’s off-the shelf compound collection (Screening Collection), as well as Enamine’s explored chemical space for drug-like structures (REAL Database™) enables an exceptionally time-efficient screening, making it possible to step forward from in-silico data set to the lead generation stage in weeks.
Hit discovery
- Customized chemical or pharmacophore diversity selection of compounds from our stock. Virtual screening selections based on molecular docking and pharmacophore modeling;
- Computational and experimental physicochemical properties assessment;
- HTS in biochemical and cell-based assays;
- Secondary assays and selectivity panels
Molecular modeling and computational biology
Our area of expertise covers focused library design and lead discovery against kinases, proteases, epigenetic targets, and protein-protein interactions.
- Molecular dynamics simulations and analysis of the conformational flexibility of biomolecules;
- Binding site identification and building pharmacophore models;
- Computational evaluation of potencies via scoring functions;
- Correction and optimization of the lead structures
Focused and targeted library design
- ADME/Tox drug-likeness profiling
- Ligand based virtual screening
- Target structure based virtual screening
- 3D Pharmacophore and shape based search
Hit explosion and lead generation
- Design and synthesis of custom libraries of structural analogs
- Generation of structure activity and property relationships (SAR and SPR)
- Scaffold hopping and replacement of unwanted structural patterns
Lead optimization
- Optimization of lead structures (biological activity – potency, selectivity, ADMET)
- Synthesis of arrays for filling gaps in SAR / SPR data
- Scaffold hopping into IP-free ligands with improved profiles
Mass spectrometry (MS) has become an important tool for high-throughput screening, owing to its ability to directly probe interactions between target proteins and ligands from the libraries of drug-like molecules. Screening by mass spectrometry, both covalent and non-covalent, includes several approaches with different areas of application. To address a growing demand of our drug discovery partners, MS-based Screening group has been established at Bienta in 2019.
Being a part of Enamine, the largest manufacturer of screening compounds for drug discovery, Bienta has gained experience in covalent screening by MS. Among the variety of the covalent probes, Cysteine-specific binders are of the highest demand. Our MS-based screening group is working with a list of Cysteine covalent fragments and screening compounds libraries, as well as custom compound selections from specific Cysteine-targeted warheads, such as acrylamides, chloroacetamides, vinyl sulfone compounds, and others. The libraries are being constantly updated, to ensure a novel and diverse chemical space for the quests for new hits.
Our team is equipped with two Agilent Q-TOF LC-MS systems dedicated for MS screening projects. A typical covalent screening project consists of 2 stages: (1) method development and (2) the screening per se, including hit confirmation and counterscreen. We mostly work with the libraries in 384-well plate format.
For the data analysis, our software team developed an in-house data parser which goes through MS peak lists from individual wells, searching for protein-ligand conjugate peaks, and calculates % of adduct formation. To satisfy all customers’ requests and answer additional questions that may arise during the screening, our MS-based Screening group can perform a variety of hit follow-up services, including diverse orthogonal screens, intrinsic reactivity, kinetic studies, and ligand binding localization studies.
At Bienta, MS-based Screening group works in close collaboration with the Recombinant Protein Expression group. Target proteins can be produced in E.coli, baculovirus or mammalian systems.
Recombinant proteins are an indispensable tool in drug discovery. The need for multiple forms of recombinant proteins, e.g. wild/mutant/fusion, from different species is increasing and we seek to fulfill the demand providing excellent service and support.
The production of recombinant proteins can be performed in:
- E.coli (multiple strains)
- mammalian cells (CHO, HEK293)
- Baculovirus-insect cells expression system
Depending on the aims of the study, we may assist in designing and producing expression constructs, or start the production of recombinant proteins with the ones provided by a customer.
To provide you with the best target for your experiments, the proteins can be tagged, biotinylated, or isolated as non-fused proteins. In addition, we offer:
- Activity measurements (biochemical or cell-based in vitro assays)
- Mass Spectrometry
- Purity assessment by SDS-PAAG, FPLC
- Thermal Shift Assay
- Evaluation of endotoxin contamination by the LAL assay
We thoroughly control the process of recombinant protein production at every step, starting from the cell transfection until measurement of a protein activity in vitro. We have all the required equipment to produce recombinant proteins of excellent quality, e.g. small-scale fermentation system, AKTA FPLC purification systems, temperature controlled incubator shakers, lyophylizers, etc. Bienta’s purified proteins exceed 90% purity as determined by SDS-PAGE, analytical chromatography and MS analysis, which would satisfy any requirements for research use.

Bienta’s state-of-the-art Laboratory Animal Research Centre was established in late 2018 to meet the escalating need for high-quality in vivo pharmacokinetic and toxicology studies in rodents. Spanning over 7000 square feet of laboratory space, our facilities are meticulously equipped and maintained, adhering to the highest industry standards.
We take the well-being of our research animals seriously and prioritize their care and comfort. Our dedicated team ensures that our animal housing conditions surpass the rigorous recommendations set forth by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). Rigorous monitoring protocols are in place to safeguard the health and welfare of our animal subjects at all times.
By upholding these stringent standards and investing in advanced facilities, we are fully equipped to conduct groundbreaking research, enabling us to provide our clients with exceptional scientific insights and reliable data.
At Bienta, we are committed to advancing the field of in vivo research through excellence, innovation, and the highest ethical standards.
Our in-house core breeding unit can produce approximately 1200 animals per month. A stock of 9 various mice and rat strains were imported and maintained, in particular:
Mice strains:
- Balb/cAnN
- C57BL/6J
- CD1(ICR)
- NMRI(Han)
- C3H/HeOuJ
- CD1-Foxn1nu
Rats strains:
- Wistar
- Sprague Dawley
- SHR
Other animals: (hamsters, guinea pigs, gerbils) are available on request.
At Bienta’s Laboratory Animal Research Centre, we prioritize the well-being and optimal living conditions of our animals. Our commitment to their comfort and safety is reflected in our meticulous housing practices.
To ensure the utmost comfort, our animals are typically housed in pairs or suitable groupings within specially designed cages. These cages are thoughtfully selected to provide a comfortable environment for our animal residents. Furthermore, we maintain strict monitoring and control measures for temperature, humidity, and light/darkness cycles to create an ideal habitat for their well-being.
The Bienta Animal Facility stands as a stronghold of protection, safeguarding both the animals and our dedicated staff from potential risks and contamination. Our facility incorporates robust air ventilation barriers to maintain a secure environment. This comprehensive approach ensures the safety and integrity of our animal subjects throughout their stay.
Our commitment to ethical animal research is ingrained in every aspect of our operations. All studies conducted at Bienta undergo a thorough review by our Institutional Bienta’s Animal Care and Use Committee (BACUC), adhering to the principles of the 3Rs (replacement, reduction, refinement). We are dedicated to upholding the highest standards outlined in the International Guiding Principles for Biomedical Research Involving Animals.
At Bienta’s Animal Facility, our team of professionals comprises biologists, geneticists, veterinarians, and skilled animal technicians. They possess the expertise necessary to provide comprehensive care for our animal residents. Their responsibilities encompass various aspects, including general animal maintenance, breeding programs, acclimatization of new arrivals, inspections, experimental support, and project coordination. This diverse skill set ensures the well-being and welfare of our animal subjects at every stage of their involvement in research.
As a testament to our commitment to scientific excellence and flexibility, we are also capable of accommodating special experimental designs by importing and maintaining additional strains as per the specific requirements and demands of our valued customers.
We offer:
- Pharmacokinetics Studies in Rodents
- Basic Formulation Screening for In Vivo Studies
- Toxicology Services
- Drug Efficacy Studies
- Aging Research
- Cancer
- Behavior
For more information, please contact us
New Technology Department was established as a natural expansion of Bienta and currently provides services for Recombinant Proteins Production and Screening by Mass Spectrometry.
New approach for solubility test is available at Bienta LTD with the Agilent 1260 Infinity that was installed at Bioanalytical Laboratory. The Agilent 1260 Infinity with Diode Array Detector equipped with OpenLab software is designed to provide high sensitivity (noise <±3µAU), and lowest peak dispersion for 2.1, 3 and 4.6 mm ID columns. Wide linear range (typically up to 2.5 AU) gives an advantage for reliable, simultaneous quantification of primary compounds, by-products and impurities. The Agilent 1260 Infinity has full spectral detection up to 80 Hz for compound identification by spectral libraries or verification of the separation quality with peak purity analysis for conventional and ultrafast liquid chromatography. Electronic temperature control provides maximum baseline stability and practical sensitivity under fluctuating ambient temperature and humidity conditions. Background interference is eliminated by reference wavelength. The optofluidic waveguides in the MaxLight cartridge cells eliminates almost any compromising refractive index and thermal effects, resulting in significantly less baseline drift for more reliable and precise peak integration which gives the Agilent 1260 Infinity highest baseline robustness.
This detector has great advantage if compare to MS/MS technique. It can detect spectra of non-ionizible compounds.

Bioanalytical Laboratory is established to provide common in vitro ADMET (absorption, distribution, metabolism, excretion and toxicity) tests as well as pharmacokinetics studies in small animals either separately or as a component of integrated drug discovery projects including screening and/or medicinal chemistry services by Enamine.
Equipment:
Five complete triple quadrupole LC-MS/MS systems (Shimadzu Prominence and Nexera X2 HPLC, AB Sciex API5000, 4000, QTRAP and API3000,) Agilent 6224 Accurate-Mass TOF LC/MS system, Agilent 1260 Infinity and all the accessory equipment needed for sample workup and analysis, including spectrophotometers/plate readers and liquid handlers for ADME automation. See ADMET and Pharmacokinetics section for a comprehensive list of services and tests.
Bioanalytical Laboratory at Bienta operates according to the standard international quality control practices and has a permit from Ukrainian regulatory agencies to provide analytical support for clinical trial PK/bioequivalence studies in humans. Bienta does not have formal international GLP certification at this time, but we operate in GLP-like manner.
rotein Thermal Shift (PTS) assay (also called Thermofluor or Differential Scanning Fluorimetry) is a straightforward biophysical method allowing detection of direct binding of tested compounds to the target protein in high-throughput screening mode. PTS is a simple, label-free HTS technology applicable to most soluble proteins, irrespectively of their functions and activities.

Bienta/Enamine offers convenient and customizable integrated services for running PTS-based HTS screens on the target protein of client’s choice as well as progressing the drug discovery projects into Hit-To-Lead and Lead Optimization phases. Our high-productivity ViiA 7 System (Applied Biosystems) allows us to screen 15 000+ compounds/week. Our PTS screening campaigns can be integrated with various attractive options:
- Since PTS is a relatively high protein consumption method, we can provide our customers with on-site recombinant protein expression and purification service;
- Affordable fee-based access to the chemically diverse Hit Selector Library of 500,000 compounds, available immediately off-the-shelf;
- Chemoinformatics and molecular modeling support for target- or ligand-based screening set selection;
- Validation of primary PTS hits in various functional/orthogonal assays;
- Quick hit expansion follow-up using Enamine’s collection of 2,000,000+ compounds;
- Integrated transition from Hit Finding into Hit-To-Lead and Lead Optimization stages employing Enamine’s Medicinal Chemistry and Bienta’s ADMET/DMPK and secondary assay support.
