During the drug discovery process, preclinical/nonclinical toxicology studies are conducted to reveal a candidate molecule’s earliest potential toxic effects as it progresses from hit to lead and ultimately to a drug candidate, reducing the number of animals involved at later stages of drug discovery. Our skilled research team tailors the toxicity study protocol to meet the unique requirements of our clients. Bienta adheres to the OECD Guidelines for the Testing of Chemicals and FELASA recommendations for working with animals, and our Institutional Animal Care and Use Committee (IACUC) approves our protocols.
Our toxicology services:
Administration routes:
- PO, IV, IP, SC, IM, IN, with food.
We perform:
- Observation for mortality, signs of toxicity, and behavioral changes at all required time points after administration;
- Weight measurement;
- Food and water intake measurements;
- Gross necropsy with macroscopic inspection;
- Clinical Chemistry panel: Alkaline Phosphatase, Alanine Aminotransferase, Aspartate Aminotransferase, γ-Glutamyltransferase, Urea, Cholesterol, Creatinine, Creatine Kinase, Total Protein, Triglycerides, Lactate dehydrogenase, Total and direct bilirubin, High-density lipoproteins, and other custom parameters;
- Hematological panel: Leukocytes (MID, Lymphocytes, Granulocytes), Erythrocytes, Platelets, Hemoglobin, Hematocrit, etc (17 parameters).
- Histopathological assessment: all the tissues included xenografted tumors (H&E staining, immunohistochemistry, other stainings on custom request both for FFPE and cryo-sections), morphometry on the custom request. Microphotographs are provided.
We provide all requested biological samples from experimental animals, such as plasma samples, flash-frozen tissues, paraffin- or cryogel-embedded tissues for histology, etc.
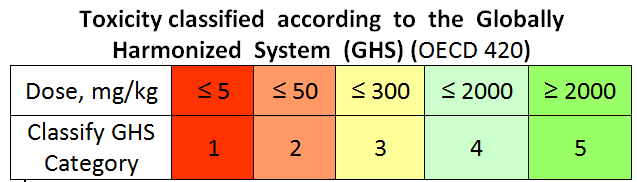
Toxicity class study
Toxicity class study is conducted to determine the class of toxicity of a substance. The results of the study help to narrow the range to search for a therapeutic dose. Fixed dose 5, 50, 300, and 2000 mg/kg are used in this study. The study requires 15 animals and 15 working days.
Dose range finding (DRF)
The dose range-finding (DRF) is an initial part of the toxicity study aimed to find the dose that will produce tolerable levels of adverse toxic effects of tested compounds. We investigate the adverse effects of acute doses administration and determine the different tolerable levels of doses – usually maximum tolerated dose (MTD), the no-observed-adverse-effect level (NOAEL) – depending on the aim of the study. Blood microsampling procedures are available to reduce the animal number. A customized DRF design is also available.
Adverse effects investigation:
- Observations for clinical signs and mortality;
- Gross necropsy with macroscopic inspection;
- Customized parameters by request (blood count, clinical chemistry, metabolic values, tissue histopathology, behavioral tests, etc.)
Deliverable: A detailed study report including the description of the study design, experimental data, and interpretation, preliminary statistics, and requested tissue samples. Raw experimental data are available upon request.
Sample Submission. Dry compound or compound in a pre-made animal dosing formulation. The amount depends on the dosing levels. For example, 1 mg of a compound will be enough for 10 mg/kg dosing.
Toxicokinetics in Rodents
The main goal of toxicokinetic studies is to establish a correlation between the compound concentration or dose and potential adverse effects and to aid in understanding the mechanisms of toxicity. Repeated dose toxicity studies in animals are performed for this purpose. Evaluation of various pharmacodynamic (adverse effects) and pharmacokinetics (absorption, biodistribution/accumulation, metabolism, excretion) parameters is usually performed after the first and last injection of the compound or drug candidate. Compound concentration in plasma is measured using the HPLC-MS/MS method. At Bienta, toxicokinetics studies are carried out in accordance with OECD 417 guidelines.
Typically, DRF is the first stage of a toxicokinetics study.
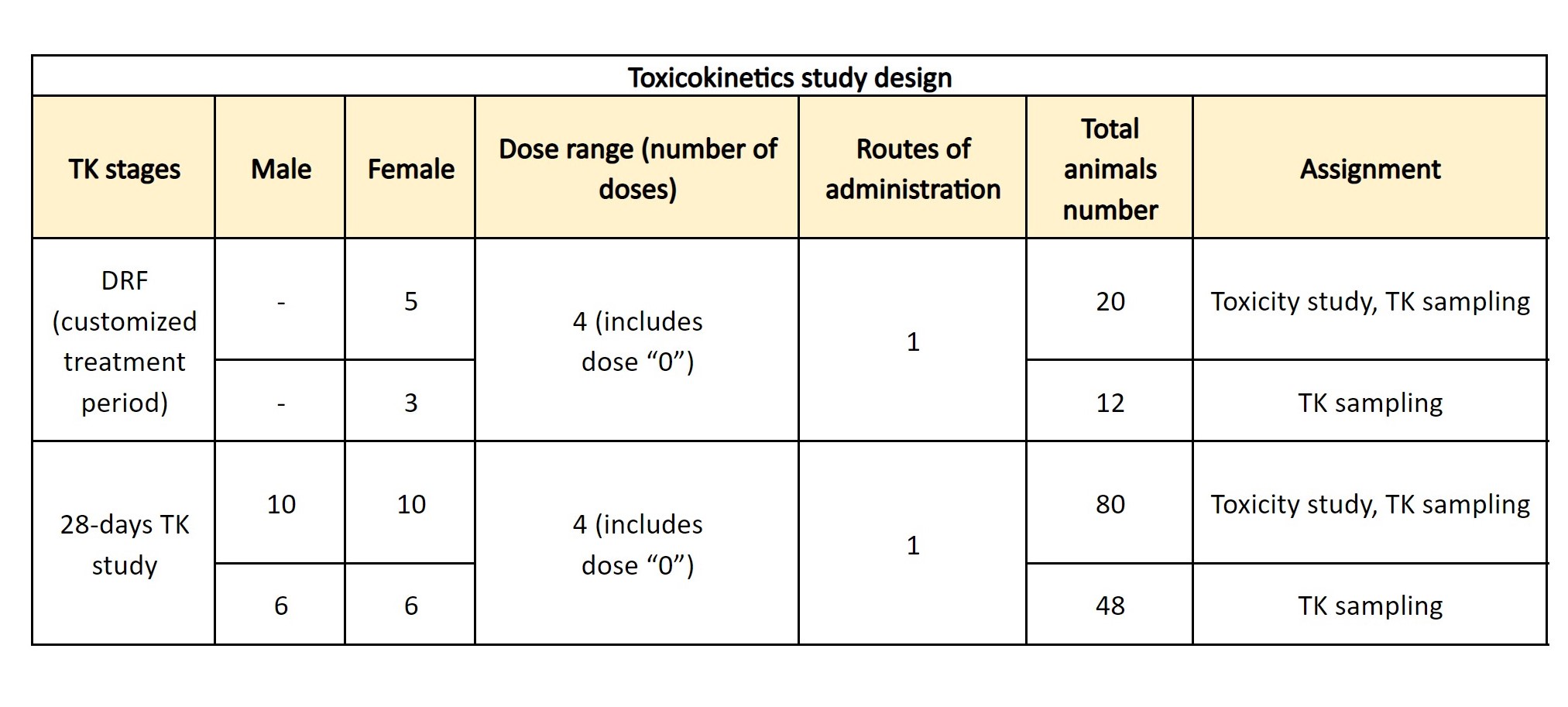
Repeated dose toxicity study
Repeated dose toxicity study is usually conducted with the aim to determine the specific dose (MTD, or NOAEL) after repeated dosage. Treatment design and duration both depend on a therapeutic treatment schedule. The study design includes 1-3 tested doses per route in animals of both sexes, 6-10 animals per group. On average, 30 animals and 30 working days are required for this study. A customized design is also available.
Pathology-specific side effects investigation:
- observations of clinical signs of toxicity
- hematology
- clinical chemistry
- behavior
- metabolic values
- customized parameters.
Deliverable: A detailed study report including a full description of study design, analytical method development, calculations of all PK parameters, and PK graphs. Raw experimental data are available upon request.
Sample Submission: Dry compound or compound in a pre-made animal dosing formulation. Amounts depend on the dosing levels. For acute toxicology study in the group of 30 mice at 10 mg/kg, approximately 10 mg of a compound is required.
Acute toxicity study
Acute toxicity studies are aimed to evaluate adverse effects after the administration of a single dose or multiple doses of a test substance given during a period not exceeding 24 hours. Acute toxicity studies are designed to determine:
- the dose that will produce either mortality or serious toxicological effects;
- time course of drug-induced clinical observations;
- the doses that should be used in subsequent studies;
- what effects the compound has on morphology, clinical chemistry, or other parameters;
- the possible target organ(s) of toxicity;
- the estimation of safe acute doses for humans.
Acute toxicity testing of potential new pharmaceutical products is traditionally conducted by at least two routes of administration: the intended clinical and a parenteral (IV or IP) route.
Service details. In the standard design, acute toxicity information is obtained from appropriately conducted dose-ranging studies that define an MTD (please, see appropriate service for details), followed by the next main phase for a more detailed study of toxic effects. In typical design in the main phase, we dose one-three groups of animals with 1-3 selected doses of the test article. The number of animals in the main phase is variable, but in general, it includes 5 rodents per sex per group. A vehicle dosing group is also included. Animals are observed for mortality and signs of gross toxicity at 30 min, 2, 4, and 6 hours after the administration and thereafter daily for a period of up to 14 consecutive days. A gross necropsy is performed on all animals at the terminal sacrifice. Specific tests, such as hematological, urinary, and blood clinical chemistry analysis, food intake, histopathology, etc., in combination with more definitive toxic or gross pathology endpoints, are available on request. The inclusion of specialized endpoints based on the pharmacology of the test article is also available.
Deliverable: A detailed study report including a full description of the study design and all experimental data. Organs and tissue samples or histological slices are also available on demand.
Sample Submission: Dry compound or compound in a pre-made animal dosing formulation. Amounts depend on the dosing levels and the design of the study.
Subacute/subchronic (repeated-dose) toxicity (MTD/NOAEL)
Subacute systemic toxicity is defined as adverse effects occurring after multiple or continuous exposure between 24 h and 28 days. Subchronic systemic toxicity is defined as adverse effects occurring after the repeated or continuous administration of a test article for up to 90 days or not exceeding 10% of the animal’s lifespan.
Service details: In the standard design, one-three groups of animals of both sexes are dosed with 1-3 pre-selected doses of a test article. Each compound-treated group as well as vehicle dosing group contains 5 animals of each sex. Animals are observed daily for signs of toxicity, body weights are recorded weekly, and food intake can be measured weekly. At the end of the experimental period, blood samples are collected for hematology and clinical chemistry analysis, and a gross necropsy is performed at the endpoint of the study. Histopathology is also available upon request.
Treatment design and duration of the research depend on a therapeutic treatment schedule. The standard study design includes 1-3 tested doses per route in animals of both sexes, 5 animals per group, and Vehicle dosed group. On average, 40 animals and 28-90 working days are required for the study. A customized design is available.
Chronic toxicity study
Chronic toxicity studies are performed for a period of 180 days to 1 year. During the study, the potential risks in relation to the anticipated dose and period of drug treatment, prospective targets of toxicity, and reversibility of clinical signs are observed.
Service details: Treatment design and duration both depend on a therapeutic treatment schedule. The standard study design includes 1-3 tested doses per route in animals of both sexes, 1 vehicle-dosed group, 20 animals per group. On average, 280 animals (including satellite groups) and 6-12 months are required for this study. Animals are observed daily for signs of toxicity, body weights are recorded weekly, and food intake can be measured weekly. Blood samples are collected for hematology and clinical chemistry analysis at selected intermediate time points and at the end of the test period. A gross necropsy is performed at all endpoints of the study. Histopathology is also available upon request. A customized design is available.
Immunotoxicity
Potential drug candidate development proceeds with the investigation of a variety of adverse effects including immune system failure. The service includes assessment of suppression or enhancement of the immune functions after treatment by testing the compound.
Service details: The service proposes various study designs to assess the suppression or enhancing the immune response in rodents, as a choice to develop hypersensitivity (allergic) reactions after exposures to the testing drug in guinea pigs. Standard study design in rodents includes investigation of hematological parameters, immune organ weights/cellularity (thymus, spleen, lymph nodes, bone marrow), serum immunoglobulins level. Specific immunological tests are available – antigen-specific plaque-forming cells testing, RBTL in vitro, immunophenotyping of leukocyte populations in the blood or lymphoid tissues, and other customized methods.
Acute Dermal Toxicity
Acute dermal toxicity study is used to identify potential side effects following skin application of a test substance. The test is performed after a single dose or multiple doses of the substance which have been applied to the skin over a 24-hour period.
Service details: In a typical design, two-five groups of female rats are dosed with 1 to 4 pre-selected doses of a test article. The test compounds are applied over the exposed area of dorsal/flank skin (~ 10 % of the total body surface area). Each compound-treated group as well as the vehicle dosing group contains 3-5 animals. Animals are observed for mortality and clinical signs of toxicity at 30 min, 2, 4, and 6 hours after the administration and thereafter daily for a period of 14 consecutive days. Individual bodу weights of animals are determined on the day of the compound administration and every two days thereafter. Gross necropsy is performed for all animals at the endpoint of the study. Specific tests, such as hematological, urine and blood clinical chemistry analysis, water and food intake, histopathology, etc. are available on request. Implementation of specialized endpoints based on the pharmacology of the test article is also available.
Infusion toxicology
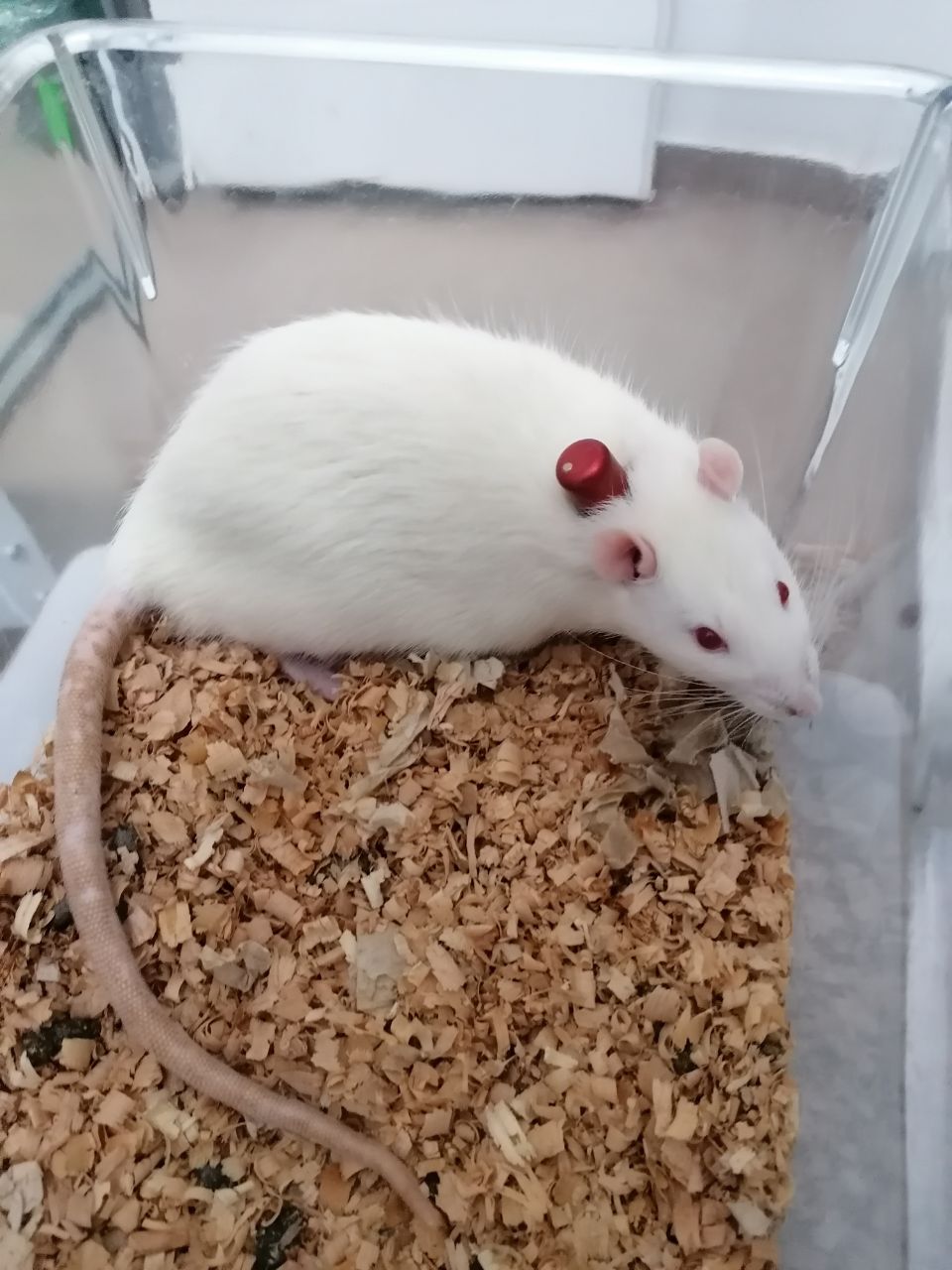
Infusion toxicology in preclinical studies are meant to asses the safety of therapeutic candidates intended for continuous administration in clinics. Slow infusions are possible through vascular access button. Bienta’s specialists possess necessary experience for the installation of the access buttons. Shortly, after the procedure animals are recovered and ready for a study.
