Chem. Eur. J. 2018, 24 (21), 5444-5449
DOI: 10.1002/chem.201800193
Kirichok A.; Shton I.; Pishel I.; Zozulya S.; Borysko P.; Kubyshkin V.; Zaporozhets O.; Tolmachev A.; Mykhailiuk P.
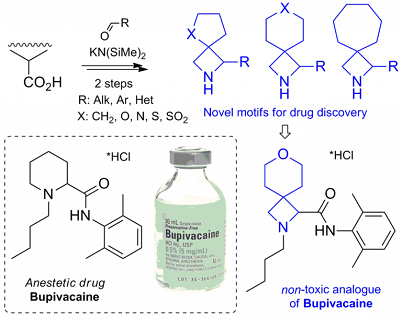
The synthesis of multifunctional spirocycles was achieved from common cyclic carboxylic acids (cyclobutane carboxylate, cyclopentane carboxylate, l-proline, etc.). The whole sequence included only two chemical steps—synthesis of azetidinones, and reduction into azetidines. The obtained spirocyclic amino acids were incorporated into a structure of the known anesthetic drug Bupivacaine. The obtained analogues were more active and less toxic than the original drug. We believe that this discovery will lead to a wide use of spirocyclic building blocks in drug discovery in the near future.