Product Focus
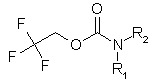
Enamine Company presents new building blocks to our valued customers. They can be used as good precursors for the preparation of unsymmetrical ureas. We have developed and synthesized a set of compounds of general formula:
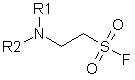
A new type of hydrolytically stable aliphatic sulfonyl fluorides matching general formula were developed and synthesized in our Company.