Product Focus
We synthesize chiral ligands for metal-based catalysts used in asymmetric synthesis:
• Chiral chelating diphosphines, phosphine-phosphinites, both known and specially designed for your purpose. These ligands are used in Rhodium or Ruthenium complexes capable to catalyse asymmetric hydrogenation of prochiral C=C and C=O bonds, hydrosilylation, hydroformylation (see, for example: Asymmetric Synthesis, (Ed.: J. D. Morrison), Academic Press, Orlando, 1985; R. Noyori, Asymmetric Catalysis in Organic Synthesis, Wiley, New York, 1994; Comprehensive Asymmetric Catalysis, (Eds.: E. N. Jacobsen, A. Pfaltz, H. Yamamoto), Springer, Heidelberg, 1999, Vol. I-III. Catalytic Asymmetric Synthesis, (Ed.: I. Ojima), Wiley-VCH, New York, 2000).
Enamine Company would like to present new Building Blocks containing CONH2 or NHCONH2 groups in side chains. These compounds also bear other functional groups allowing easy transformations to desired screening compounds with primary amide and ureide moieties.
Quite often hydrogen bonding interaction of a ligand amide group with a protein has its functional consequences. To a great extend protein - ligand binding occurs via hydrogen bonding. In the search for new hits and leads it is very important in combinatorial synthesis to use the least possible number of synthetic steps. Reactions of sulfonic acid chlorides are well studied and quite often used in nucleophilic substitution reaction.
Current reagent is an effective and facile generator of difluorocarbene. Phenyl(trifluoromethyl)mercury is a stable, crystalline solid which releases CF2 under mild, nonbasic reaction conditions. The compound is hydrolytically stable and can be handled under standard laboratory conditions. The reaction of PhHgCF3 and 3 molar equiv of anhydrous sodium iodide in benzene medium in the presence of olefins serves excellently in the synthesis of gem-difluorocyclopropanes. High products yields could be obtained in reaction times of about 15hr at 80-85°C.
Piperazines are essential elements in drug design. Currently MDL© Drug Data Report database contains over 11 800 structures bearing the piperazine moiety. Enamine would like to present our set of exclusive "small" piperazines - compounds with low molecular weight (below 190 Da).
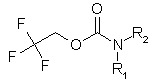
Enamine Company presents new building blocks to our valued customers. They can be used as good precursors for the preparation of unsymmetrical ureas. We have developed and synthesized a set of compounds of general formula:
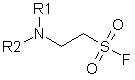
A new type of hydrolytically stable aliphatic sulfonyl fluorides matching general formula were developed and synthesized in our Company.