Tetrahedron , 2010, 66 (32), 5982-5986
DOI: 10.1016/j.tet.2010.06.036
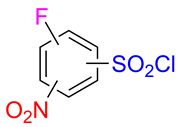
The synthesis of five hitherto unknown isomeric fluoronitrobenzenesulfonyl chlorides is described. The compounds are prepared from difluoronitrobenzenes by a two-step procedure. In the first step the starting compounds undergo a regioselective reaction with phenylmethanethiol giving rise to the corresponding thioethers. The oxidative cleavage of the latter with chlorine results in the sulfonyl chlorides in good yields. One example of a threefold sequential functionalization of 2-fluoro-6-nitrobenzenesulfonyl chloride showing the synthetic utility of the title compounds is provided.
Zhersh S.; Lukin O.; Matvienko V.; Tolmachev A.
Tetrahedron 2010, 66 (32), 5982-5986
DOI: 10.1016/j.tet.2010.06.036
