Bioorg. Med. Chem. Lett. 2015, 25 (16), 3105‑3111
DOI: 10.1016/j.bmcl.2015.06.018
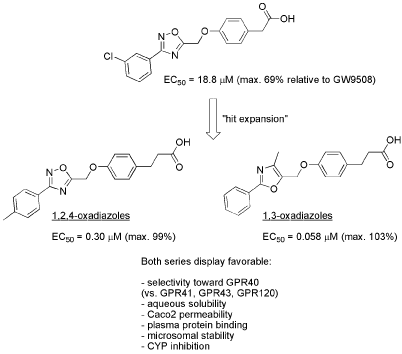
A screening hit that showed a weak (EC50 = 18 μM), partial agonistic effect on GPR40 was used a prototype for expedited hit expansion effort using a set of advanced building blocks. The latter yielded several 1,3-oxazoles and 1,2,4-oxadiazoles with significantly improved potency (best EC50 = 0.058 μM). The lead compounds in each chemotype showed a very good ADME profile (aqueous solubility, plasma protein binding, microsomal stability and membrane permeability) and no appreciable inhibition of key cytochromes P450. The compounds reported are significant new starting points for further preclinical development of future diabetic agents with a mechanism of action for which a first-in-class agent is yet to be approved.