Synthesis , 2012, 44 (08), 1263-1267
DOI: 10.1055/s-0031-1290808
Geraschenko O. V.; Khodakovskiy P. V.; Shivanyuk O. N.; Shishkin O. V.; Mykhailiuk P. K.; Tolmachev A. A.
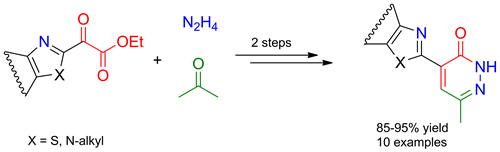
Ethyl azol-2-ylglyoxylates react smoothly with acetone in the presence of catalytic amounts of proline and trifluoroacetic acid to give the corresponding azolyl aldols in 93-98% yield. The products are easily transformed into azolyl pyridazinones in 85-98% yield by cyclization with hydrazine hydrate.
Geraschenko O. V.; Khodakovskiy P. V.; Shivanyuk O. N.; Shishkin O. V.; Mykhailiuk P. K.; Tolmachev A. A.
Synthesis 2012, 44 (08), 1263-1267
DOI: 10.1055/s-0031-1290808
