J. Comb. Chem. , 2008, 10 (6), 858-862
DOI: 10.1021/cc800074t
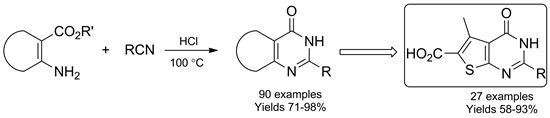
The parallel solution-phase synthesis of substituted thieno[2,3-d]pyrimidin-6-carboxylic acids has been accomplished. This strategy relies on a cyclization of 2-aminothiophen-3,5-dicarboxylates with a set of nitriles, followed by hydrolysis to construct the library of corresponding acids. The convenient procedure for use and dosage of dry HCl for the reaction was elaborated and adapted for semiautomated solution-phase parallel synthesis. With the use of another (hetero)aromatic ortho-aminocarboxylate, mini-libraries of diverse fused pyrimidin-4-ones were synthesized. The scope and limitations of the approach are discussed.
Bogolubsky A. V.; Ryabukhin S. V.; Plaskon A. S.; Stetsenko S. V.; Volochnyuk D. M.; Tolmachev A. A.
J. Comb. Chem. 2008, 10 (6), 858-862
DOI: 10.1021/cc800074t
