Eur. J. Org. Chem. 2015, 4, 886-891
DOI: 10.1002/ejoc.201403295
Iminov R. T.; Mashkov A. V.; Vyzir I. I.; Chalyk B. A.; Tverdokhlebov A. V.; Mykhailiuk P. K.; Babichenko L. N.; Tolmachev A. A.; Volovenko Y. M.; Biitseva A.; Shishkin O. V.; Shishkina S. V.
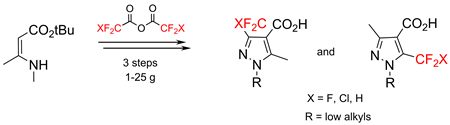
Acylation of tert-butyl 3-(methylamino)but-2-enoate with fluorinated acetic acid anhydrides occurred at the enamine carbon atom. The reaction of the resulting tert-butyl 3-(methylamino)-2-(RFCO)but-2-enoates with alkyl hydrazines resulted in mixtures of isomeric pyrazoles that were easily separated by column chromatography. The target fluorinated pyrazole-4-carboxylic acids were obtained on a multigram scale.