Cent. Eur. J. Chem. 2014, 12 (1), 67-73
DOI: 10.2478/s11532-013-0344-y
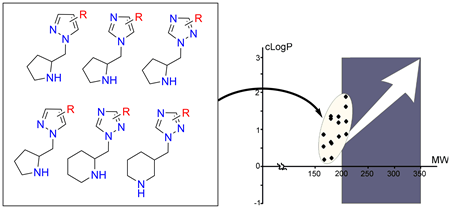
A convenient preparation of 1-(pyrrolidin-2-yl)-1H-pyrazoles, -imidazoles, and -1H-1,2,4-triazoles, 1-(piperidin-2-yl)-1H-pyrazoles and -1H-1,2,4-triazoles, and 1-(piperidin-3-yl)-1H-1,2,4-triazoles by alkylation of azoles (viz. pyrazoles, imidazoles, and triazoles) with N-Cbz-prolinol mesylate or its analogues and subsequent deprotection is reported. The two-step method allows for synthesis of the title compounds in 16-65% yields. The utility of the procedure has been demonstrated by multigram preparation of a 15-member building block mini-library for the lead-oriented synthesis of compound libraries. These building blocks perfectly fit the definition of low-molecular-weight hydrophilic three-dimensional templates, which leave much room for the lead-oriented synthesis of the compound libraries.