Tetrahedron Lett. , 2012, 53 (30), 3847-3849
DOI: 10.1016/j.tetlet.2012.05.020
Tymtsunik A. V.; Bilenko V. A.; Ivon Y. M.; Grygorenko O. O.; Komarov I. V.
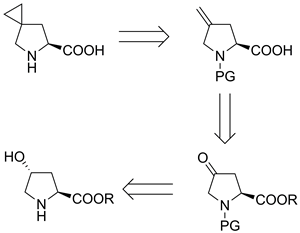
The synthesis of the Boc derivative of a novel member of the cyclopropane-modified proline library, Boc-protected 5-azaspiro[2.4]heptane-6-carboxylic acid, is reported. The synthesis was performed in six steps starting from (2S,4R)-4-hydroxyproline using a modified Simmons–Smith reaction as the key step. The reaction conditions for all the steps were carefully selected to avoid racemization at the chiral centers in the intermediates and the final product.
Tymtsunik A. V.; Bilenko V. A.; Ivon Y. M.; Grygorenko O. O.; Komarov I. V.
Tetrahedron Lett. 2012, 53 (30), 3847-3849
DOI: 10.1016/j.tetlet.2012.05.020
