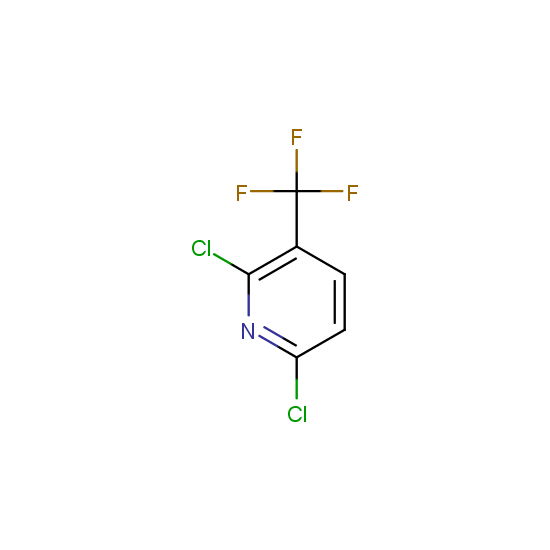
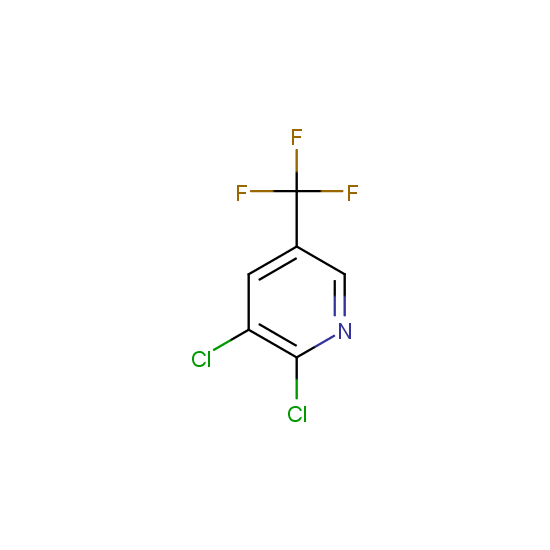
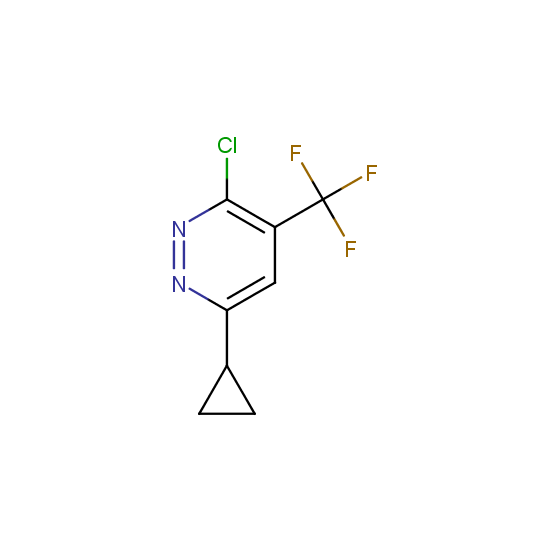
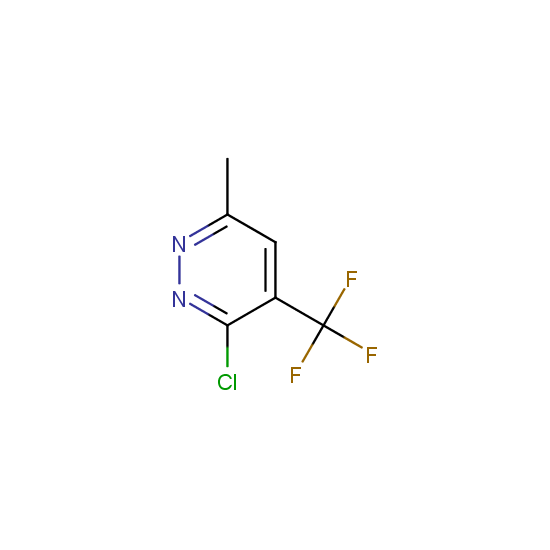
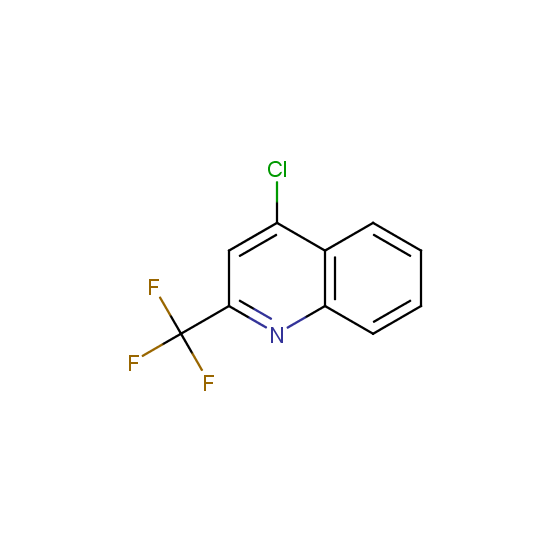
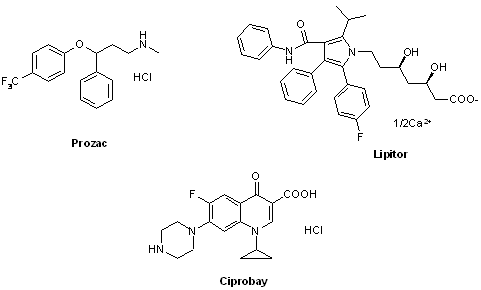
The number of commercial pharmaceutical compounds which contain at least one fluorine substituent noticeably increased since 1950th, when the first fluorine-containing drugs were introduced. At present, about 20% of the commercial drug substances are fluorine-substituted organic compounds. As the prominent examples, top-selling anti-depressant Fluoxetine (Prozac), the cholesterol-lowering drug Atorvastatin (Lipitor), the antibacterial Ciprofloxacin (Ciprobay) could be named.
In this issue, we present Fluorine-Substituted Building Blocks - a selection from the current stock of Enamine Building Blocks.
Noteworthy, Ukrainian chemists were among those who pioneered the field of organofluorine chemistry. The work in this area started in Institute of Organic Chemistry of the Ukrainian Academy of Science in early 1950th by Lev Yagupolskii and his co-workers and culminated in the development of Riluzole in 1963 - a drug used to treat amyotrophic lateral sclerosis. Much experience was gained by the Ukrainian chemists in the field since then, which is advantageously used today to synthesize new products for drug discovery.

Understanding the importance of the fluorine-substituted compounds for the pharmaceutical industry, we put much effort in adapting known and developing new synthetic methodologies allowing fluorine introduction by the use of different fluorine reagents. Apart from classical perfluoroalkyl-containing reagents (e.g. Ethyl trifluoroacetate, Trifluoroacetaldehyde, 1,1,1-Trifluoroacetone, 1,1,1-Trifluoroacetylacetone, Ethyl 1,1,1-trifluoroacetoacetate etc.) the arsenal of starting materials includes:
- DAST, MorphDAST and Sulfur tetrafluoride;
- Hydrogen fluoride;
- Metal fluorides and TBAF;
- Tetrafluoroboric acid (for Schiemann process);
- Trifluorotrimethylsilane;
- Trifluoromethyl iodide;
- Trifluoromethyl diazomethane;
- Phenyl(thifluorometyl)mercury;
- Trifluoroacetonitrile;
- Perfluoropropene and its epoxide.
The compounds obtained in such way are original, in many cases unavailable from any other supplier. Many of them were obtained by stereoselective routes; therefore, they are enantiomerically pure or enriched. The procedures employing the reagents mentioned above allow production of original fluorine-containing building blocks at 1–10 g scale; novel compounds of the requested structure can be obtained in 4–8 weeks.
Enamine chemists have developed many synthetic procedures allowing synthesis of isomeric sets containing -F, -CF3, -CHF2, -OCF3, -SCF3, -CF2- groups. These isomeric sets are particularly useful in SAR studies.
Below, we show some examples of diverse Fluorine-Substituted Building Blocks set. Complete Enamine Fluorine-Substituted Building Blocks selection (more than 2400 compounds) is available at Databases section of our site.
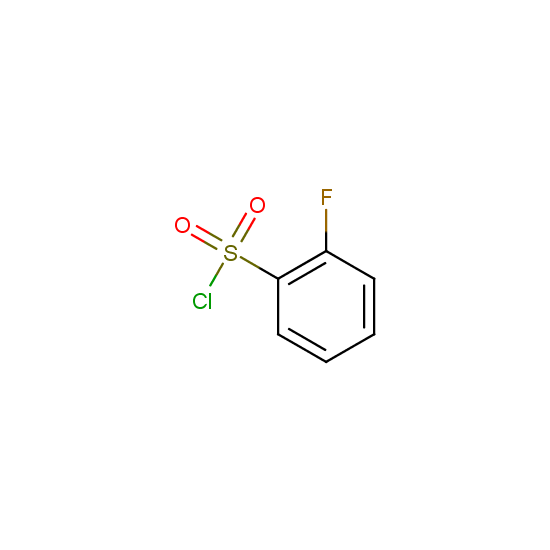
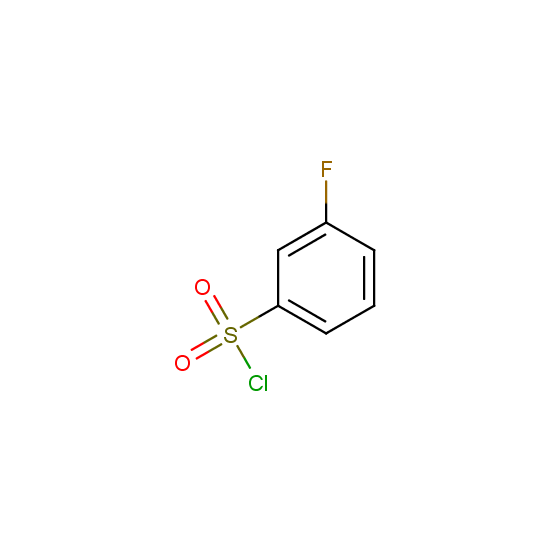
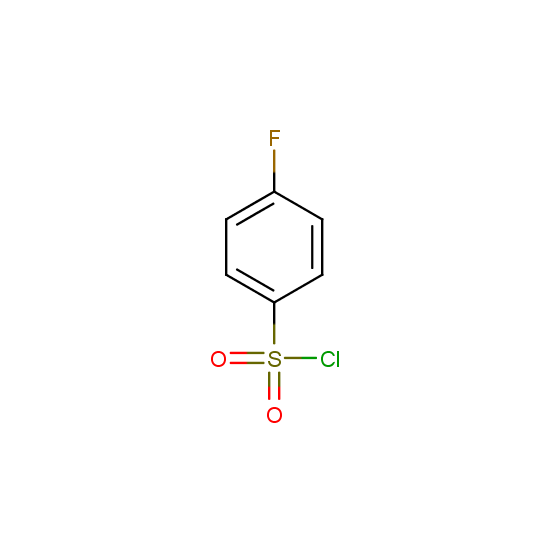
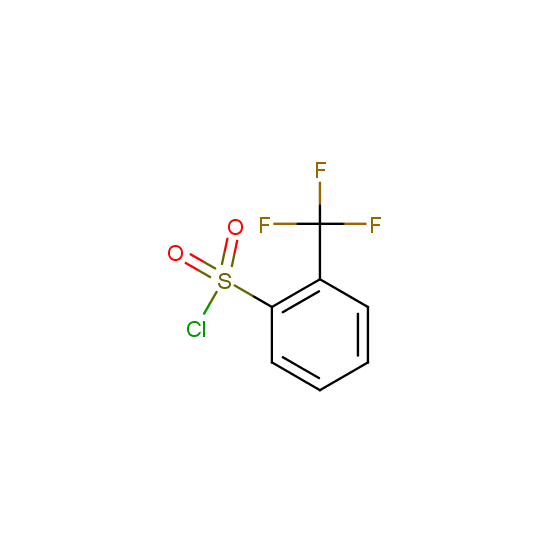
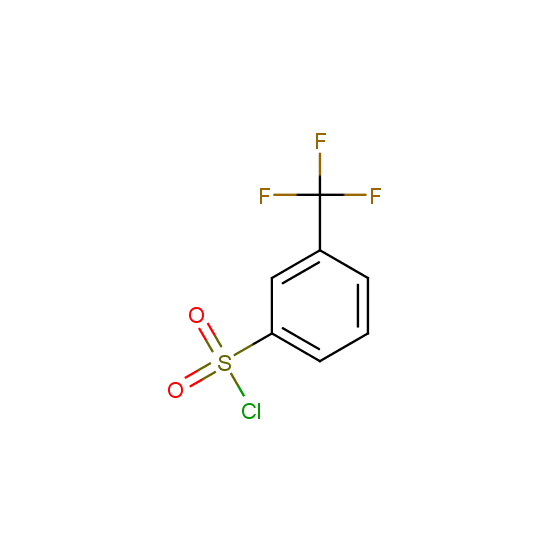
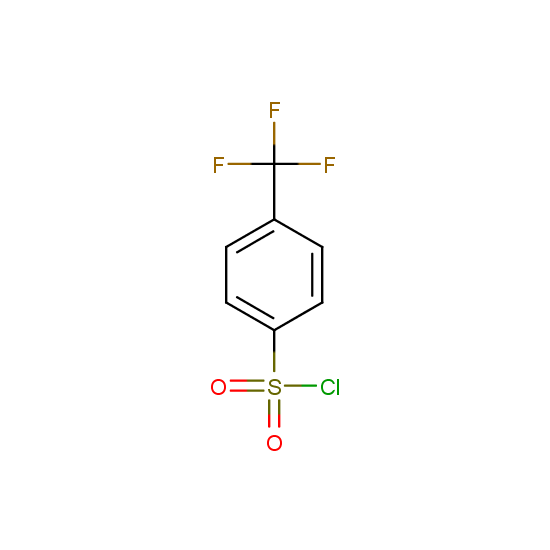
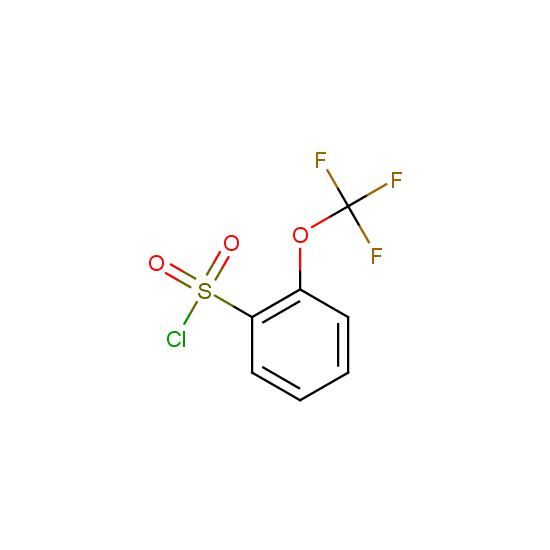
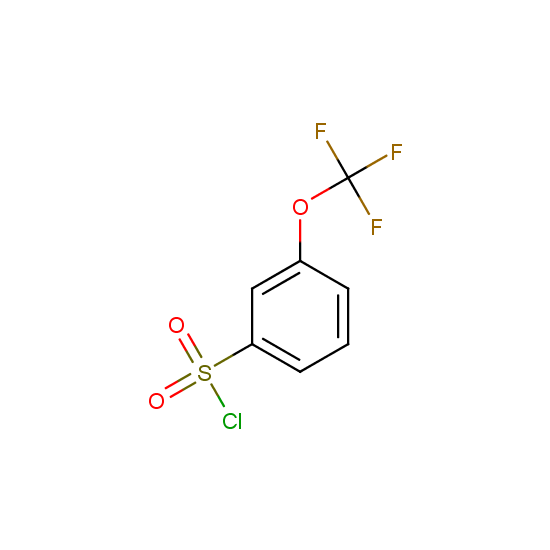
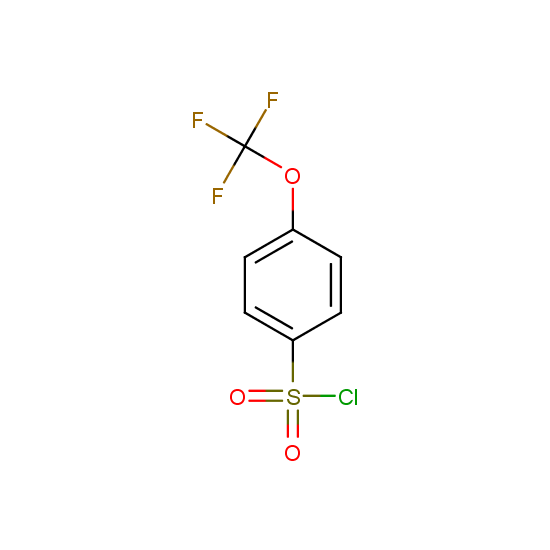
For example, all possible isomers of the sulfochlorides shown below are available:
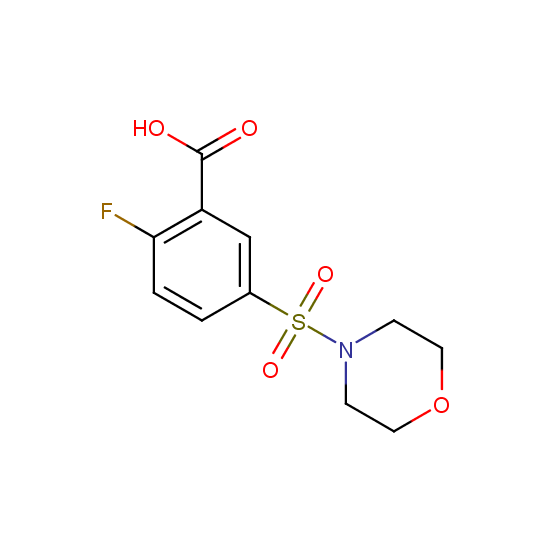
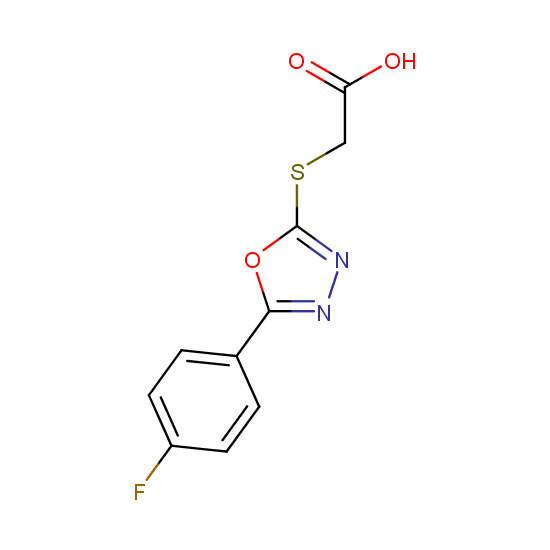
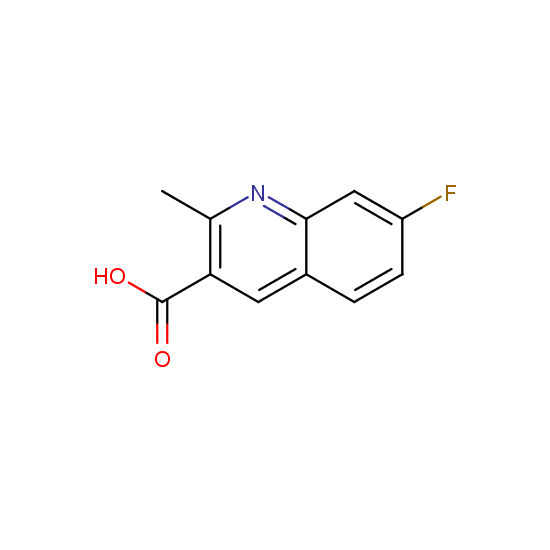
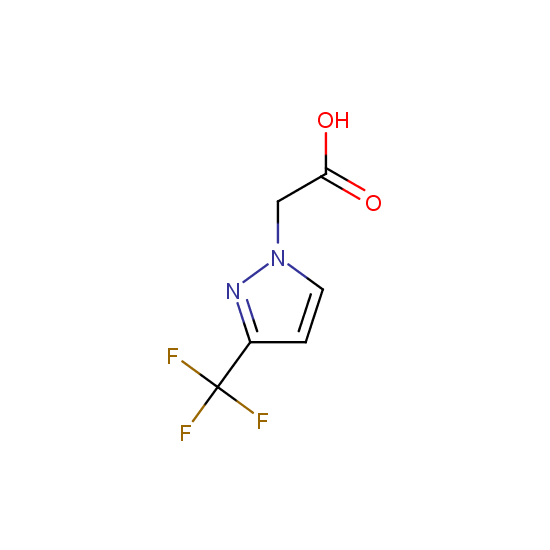
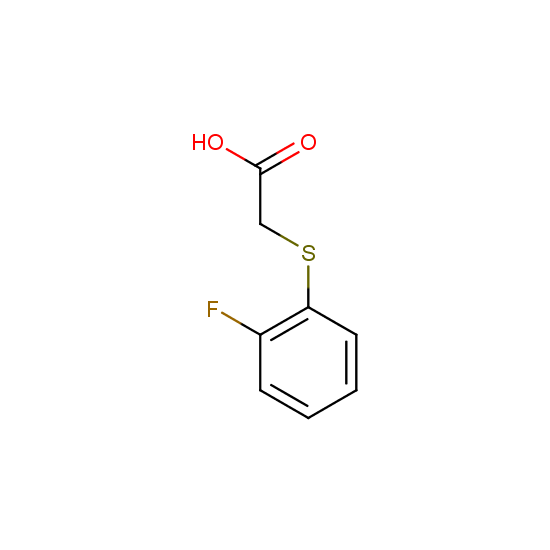
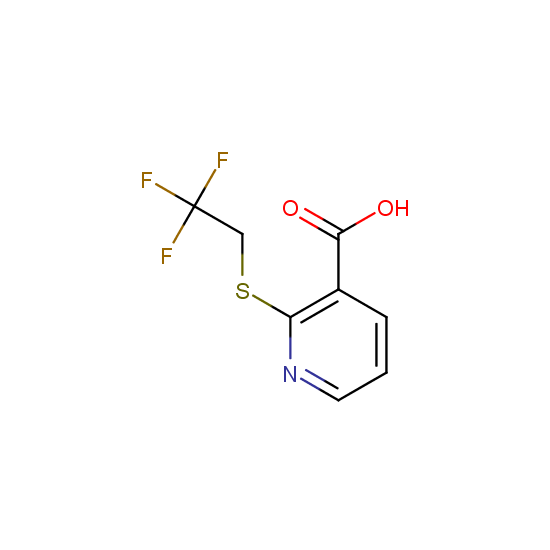
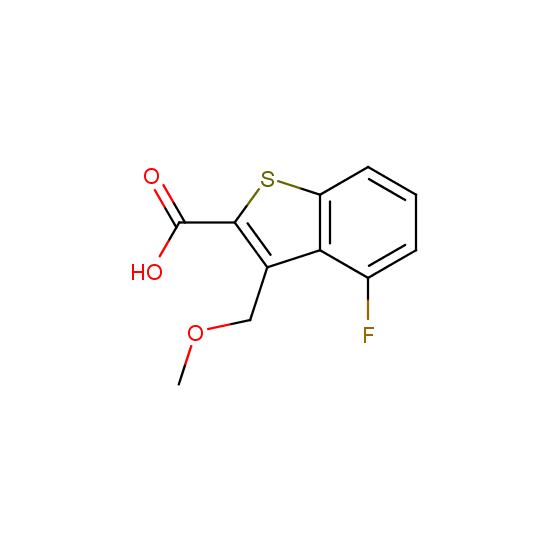
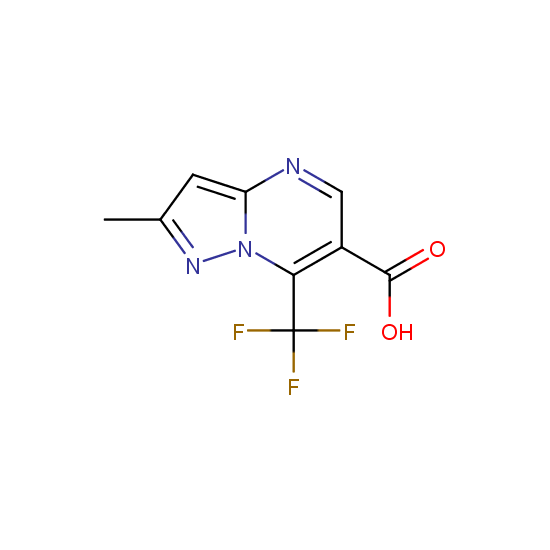
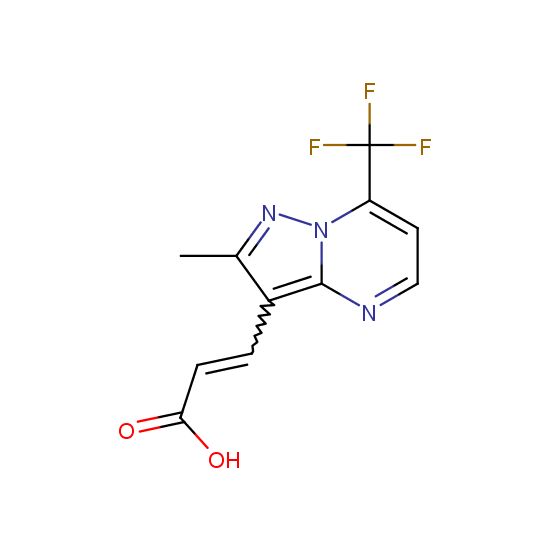
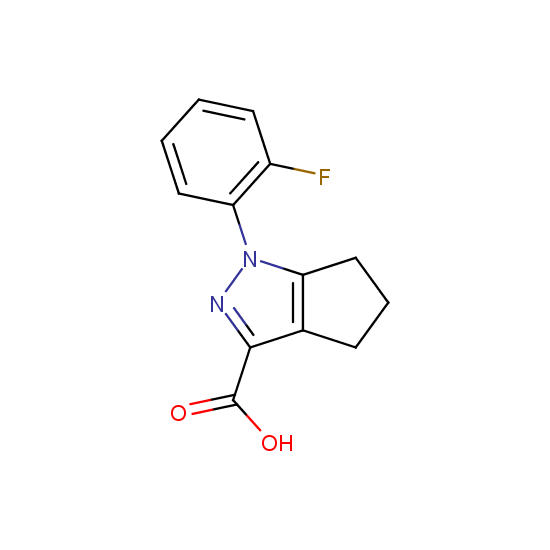
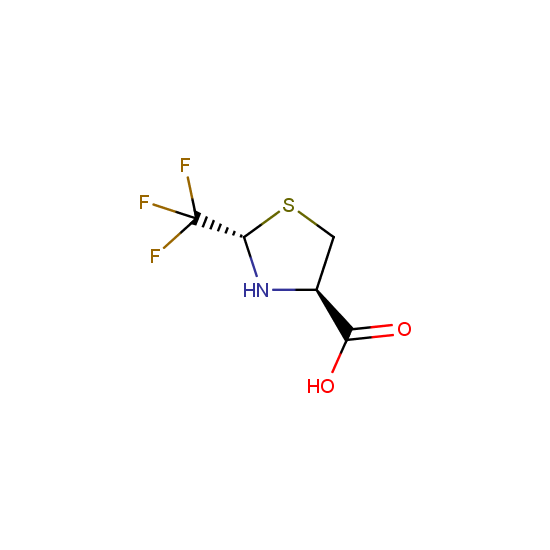
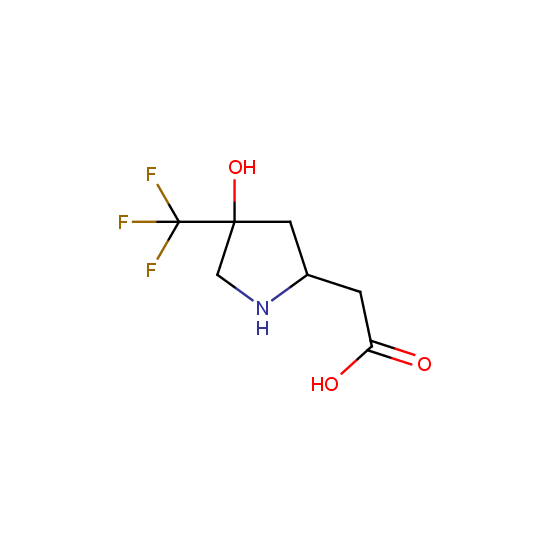
Apart from the –SO2Cl group, other functional groups in the building blocks can be used for further functionalization, for example, the carboxylic group:
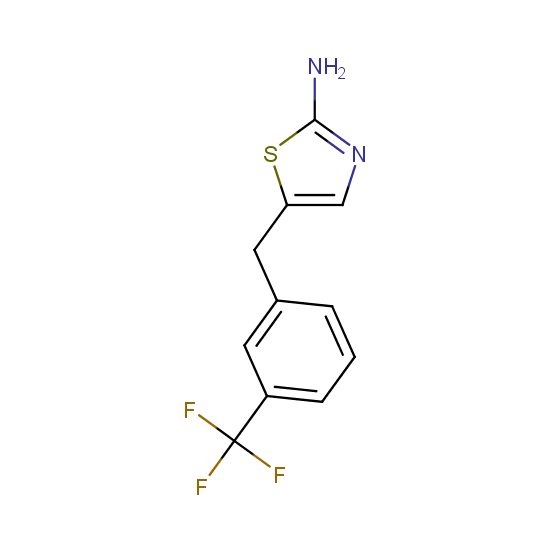
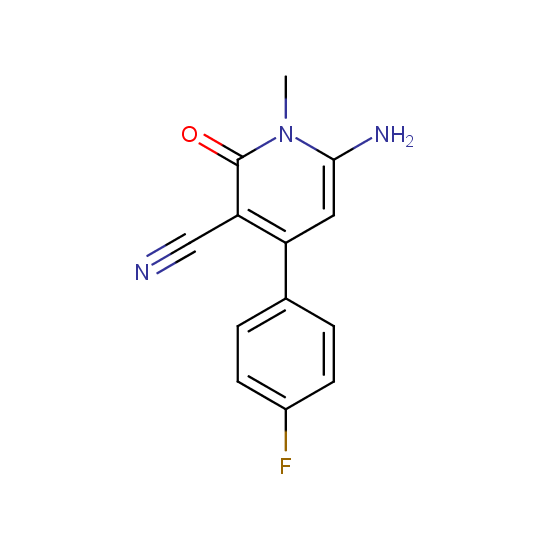
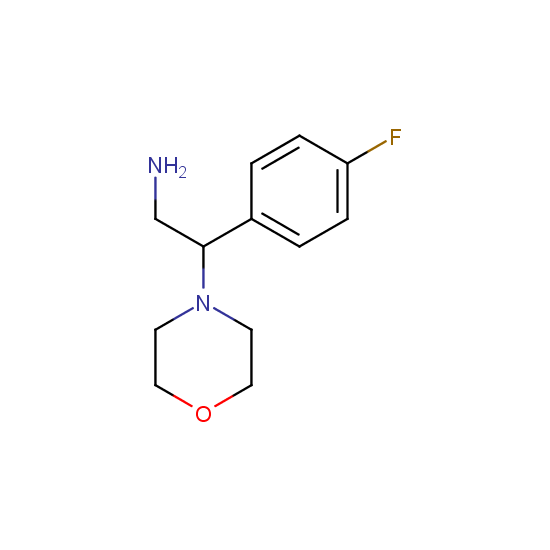
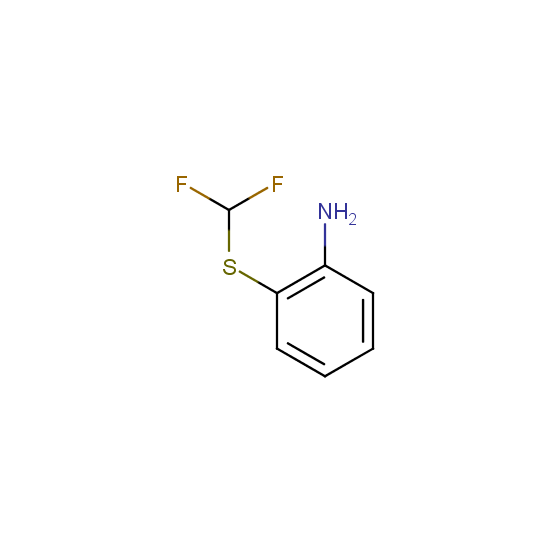
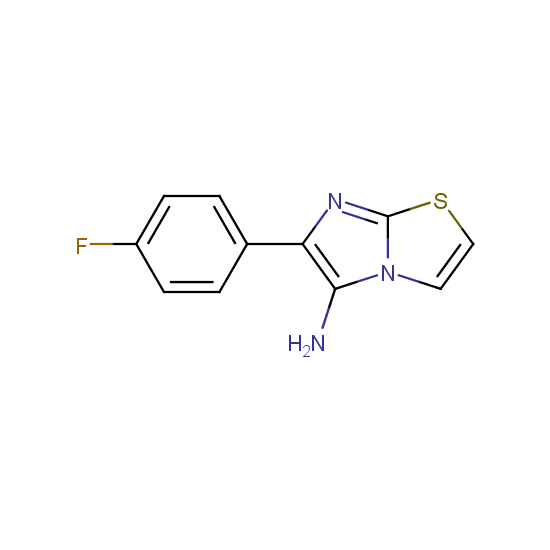
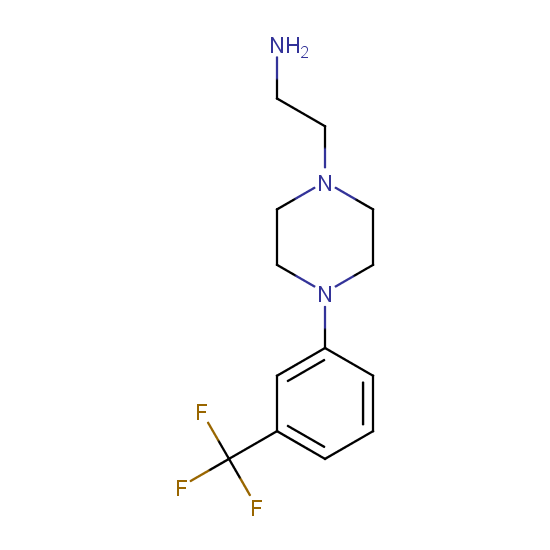
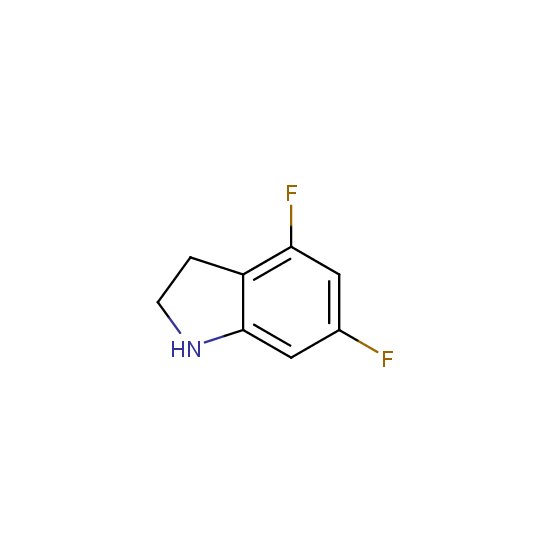
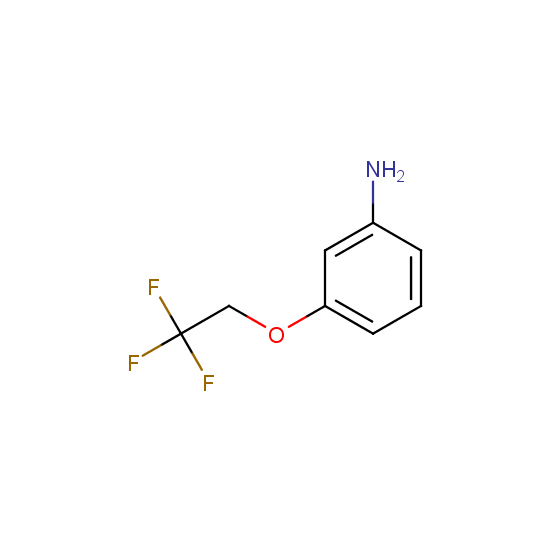
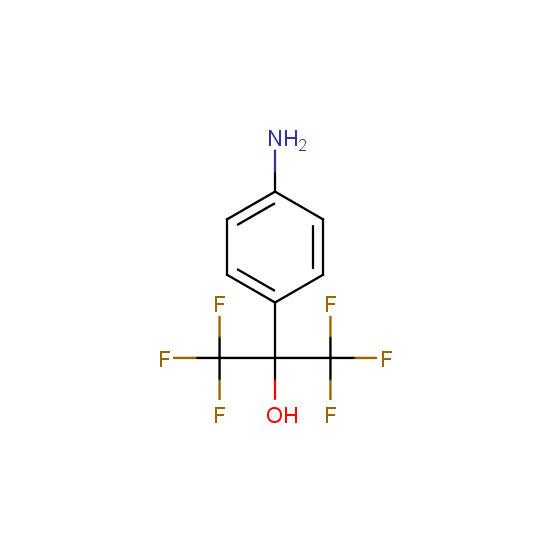
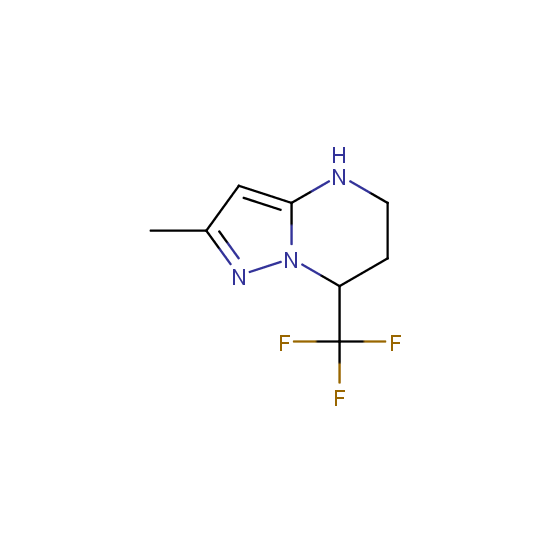
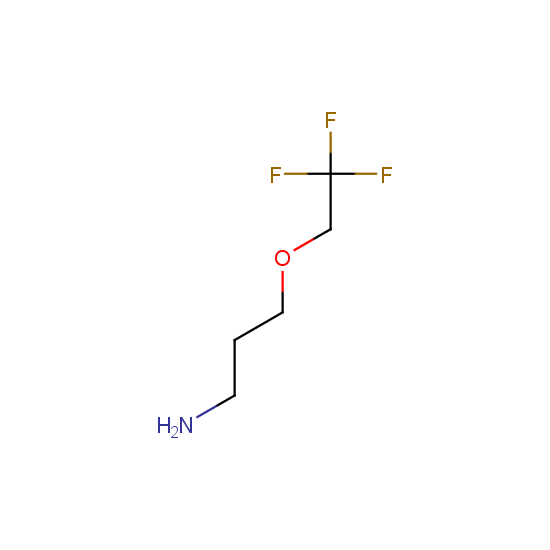
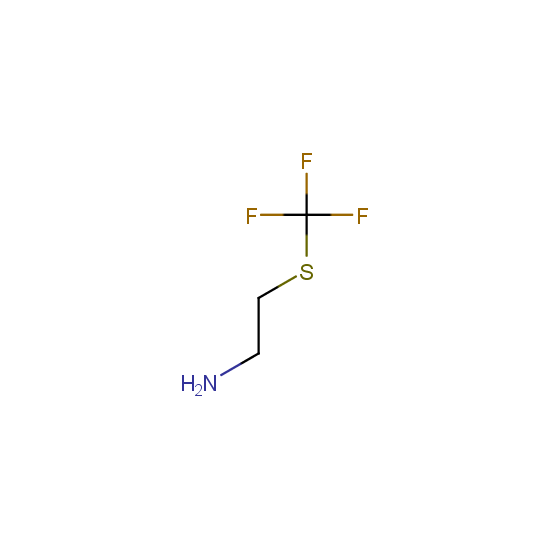
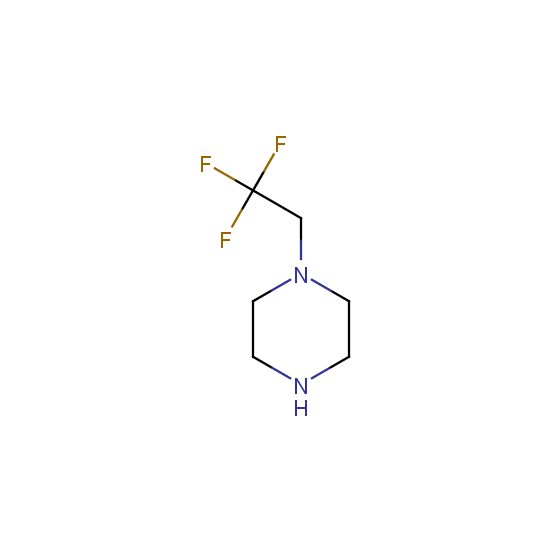
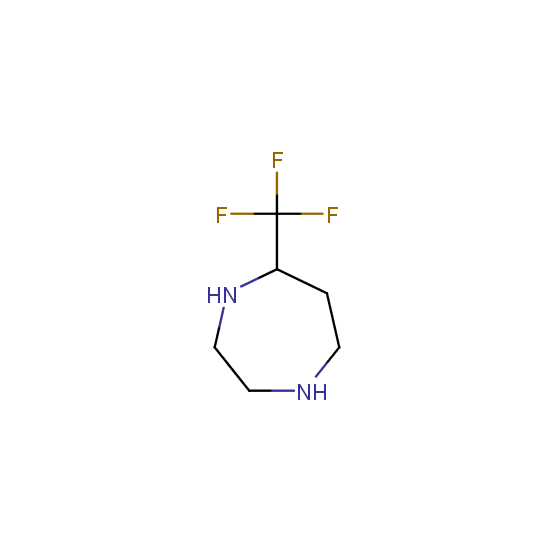
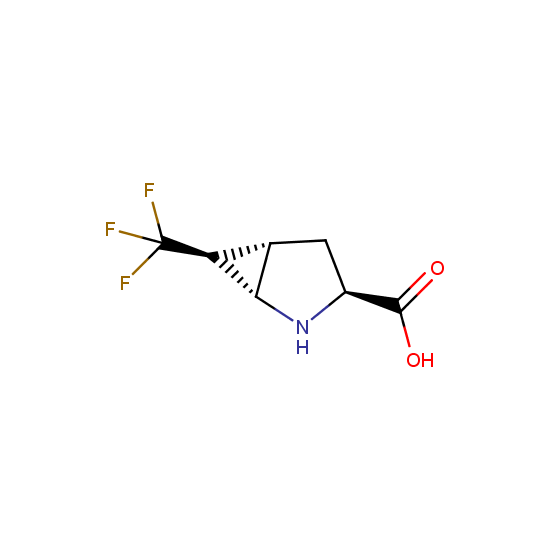
Amino group is present in fluorinated building blocks as well:
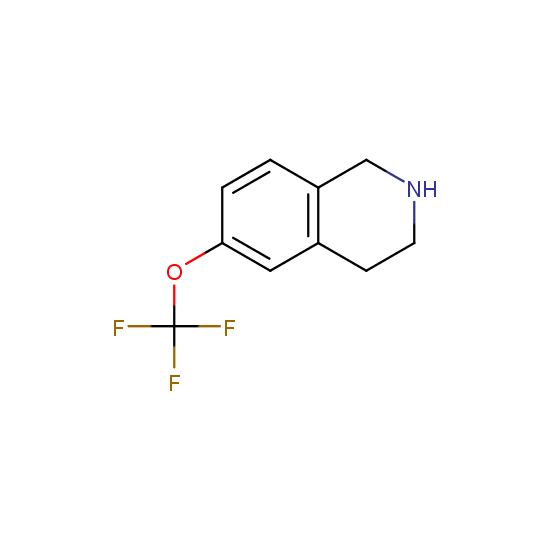
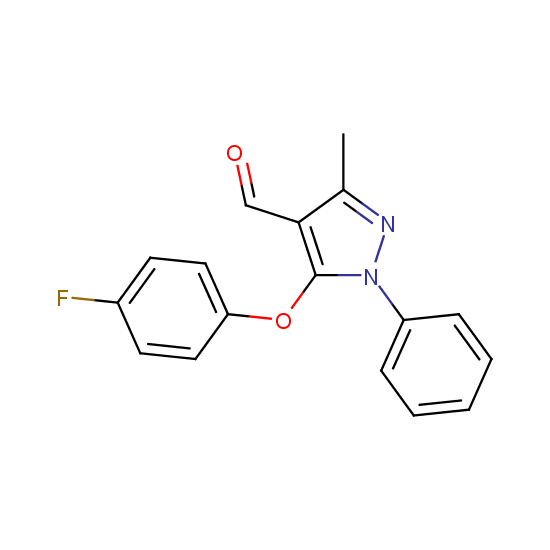
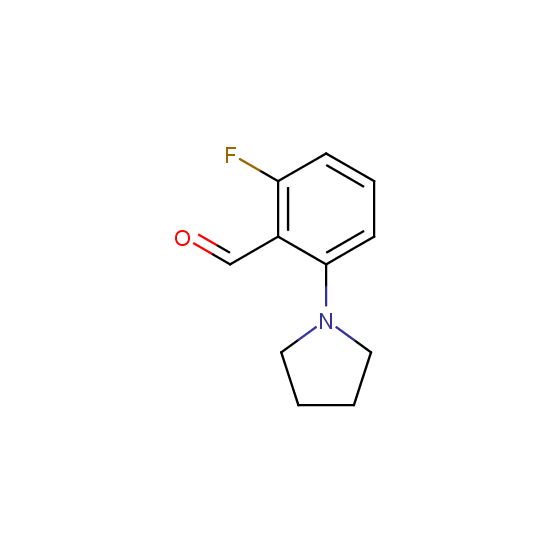
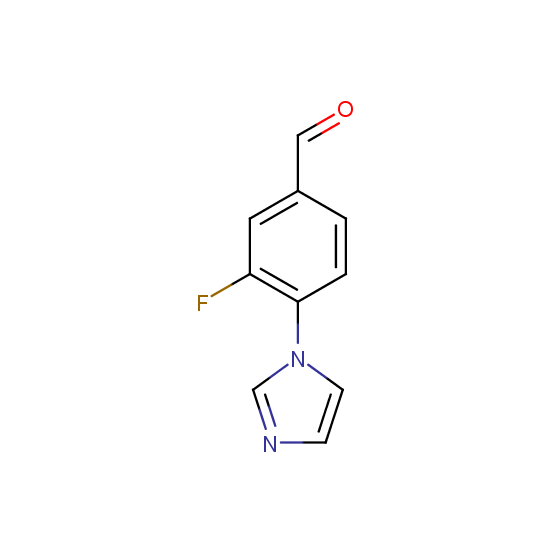
A number of building blocks with aldehyde functionality are available:
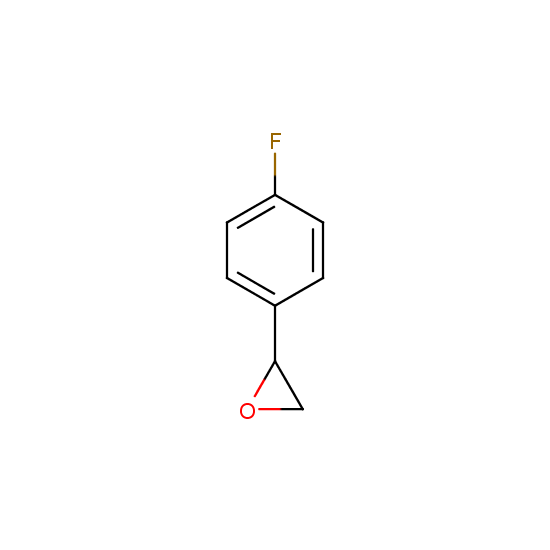
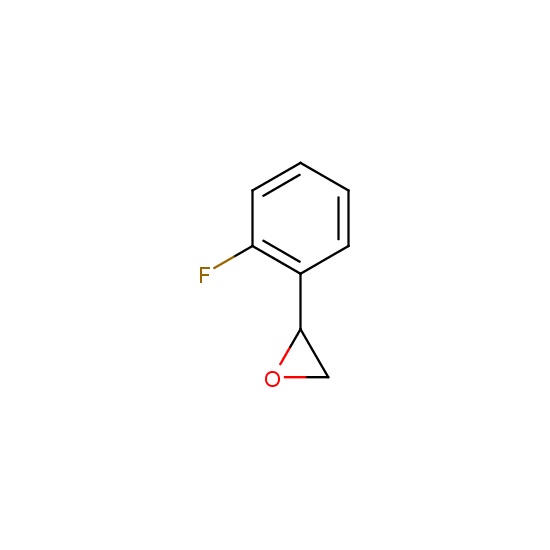
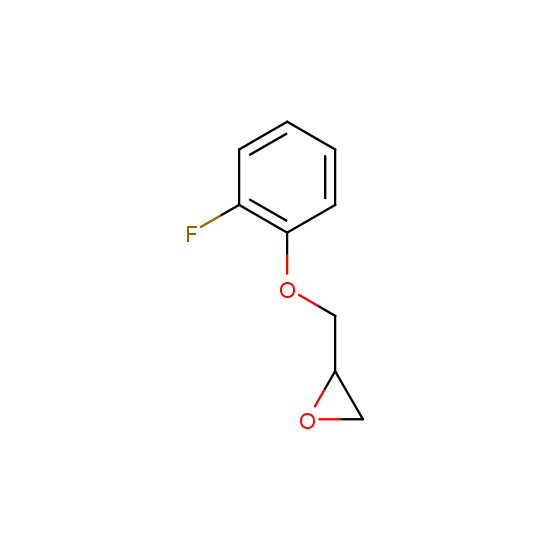
Epoxide moiety is also introduced in some fluorinated building blocks:
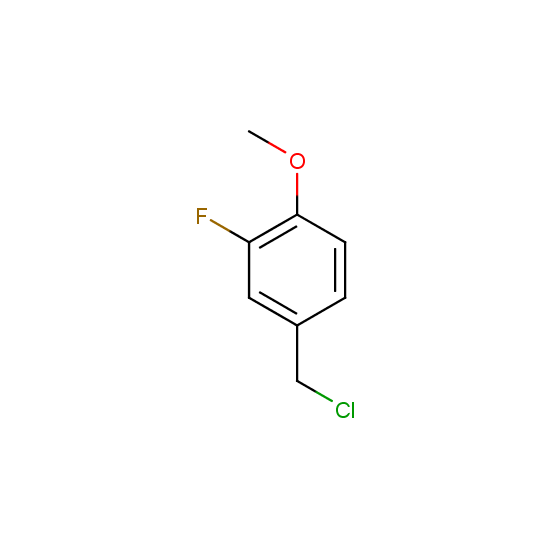
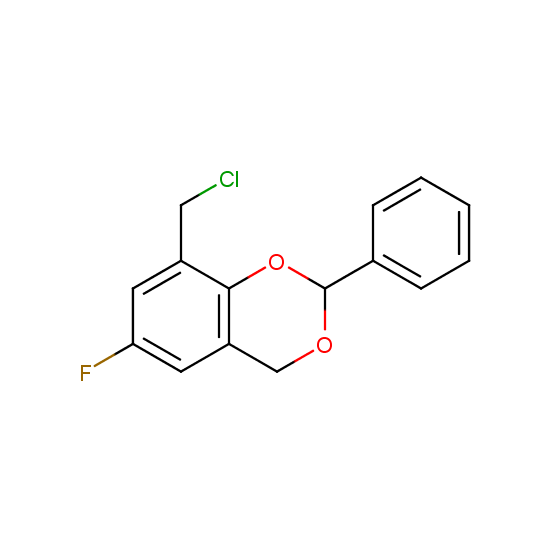
Alkylating and arylating agents with fluorine substituents are offered:
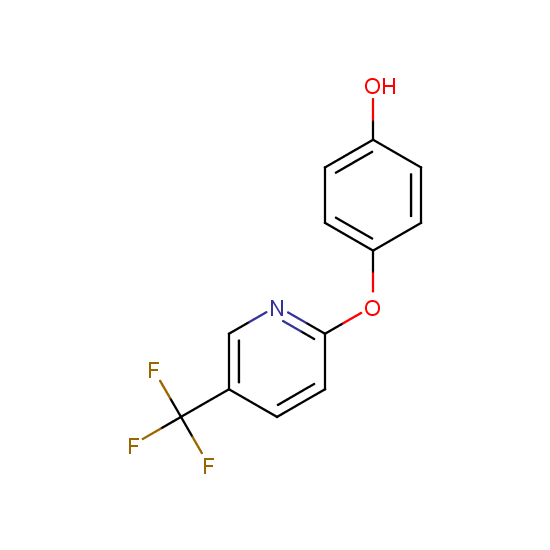
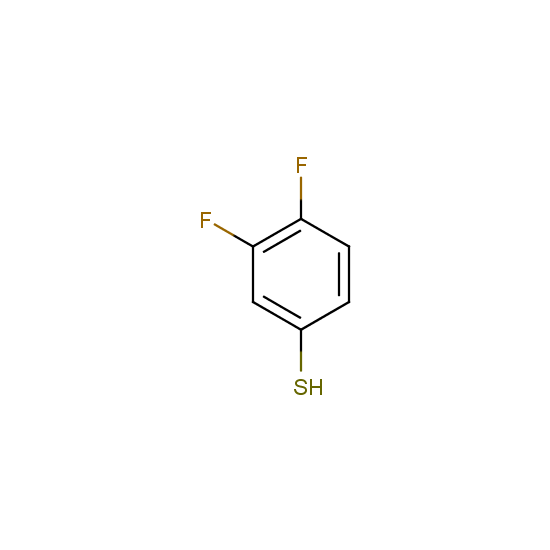
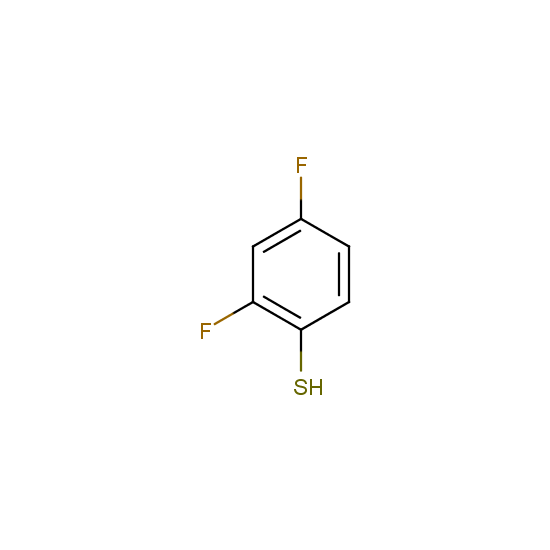
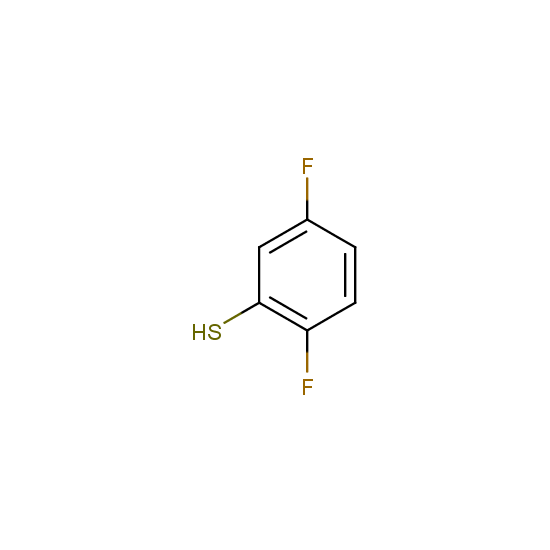
Selected thiols, phenols and alcohols with are included in Enamine Fluorine-Substituted Building Blocks:
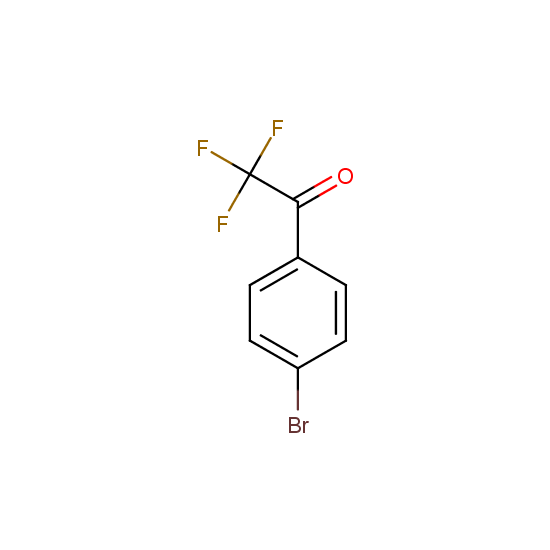
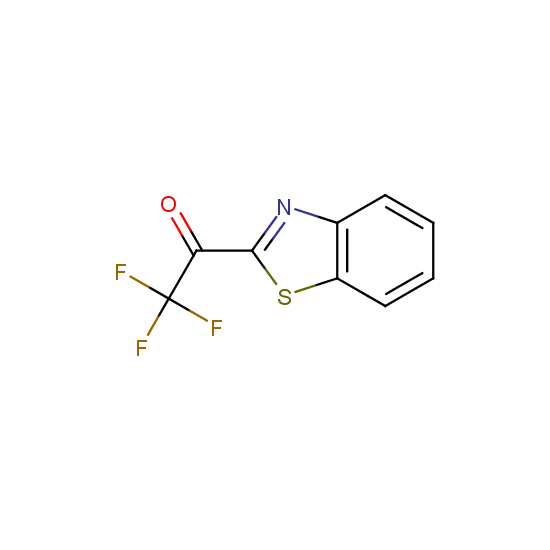
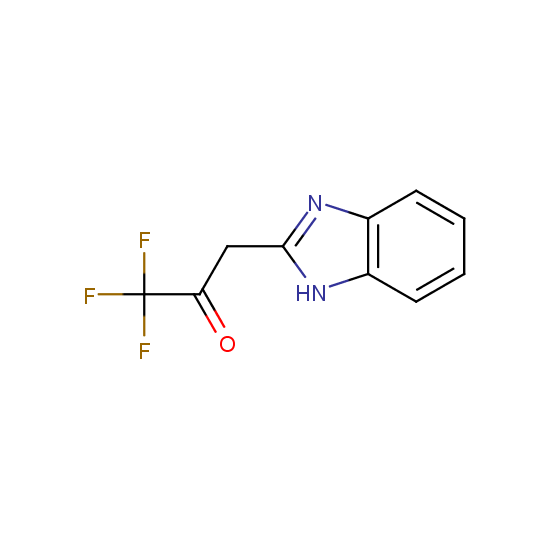
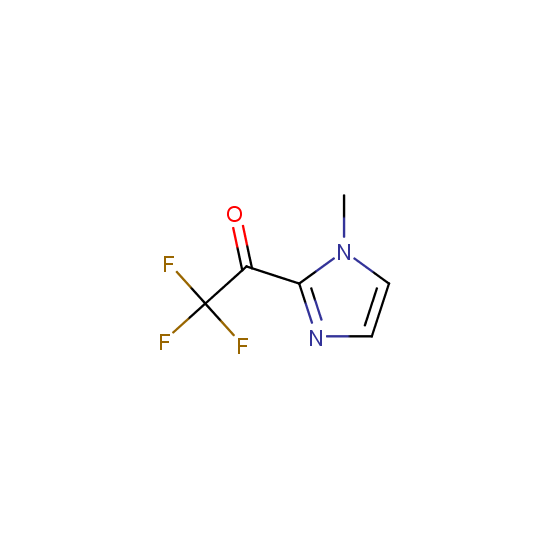
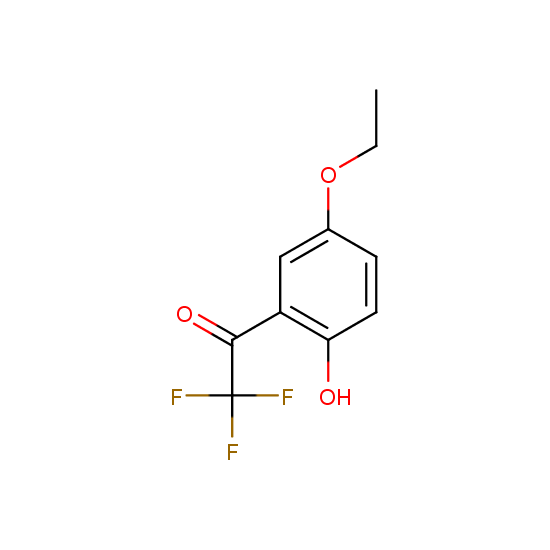
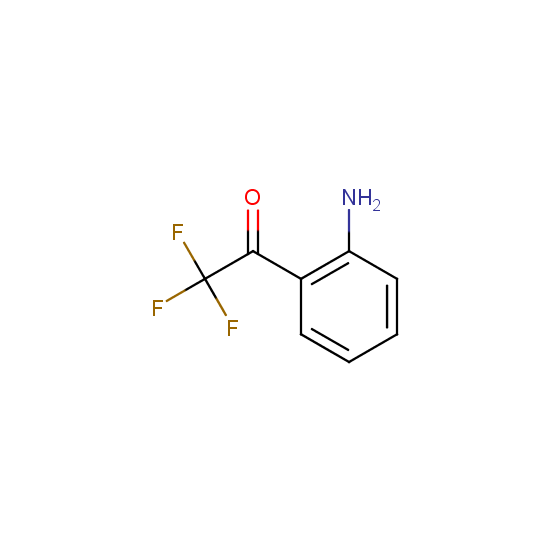
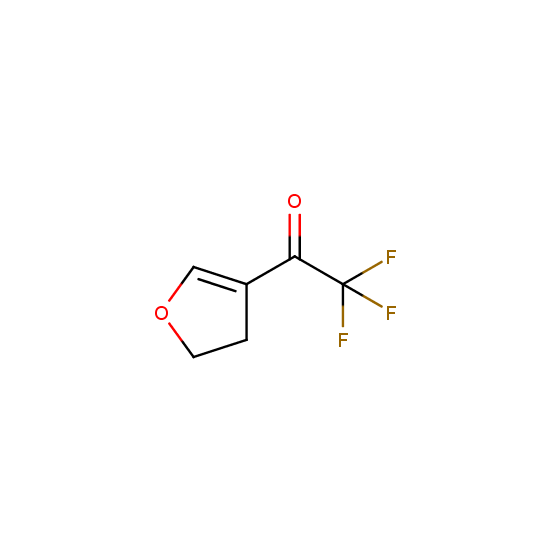
Trifluoromethyl ketones:
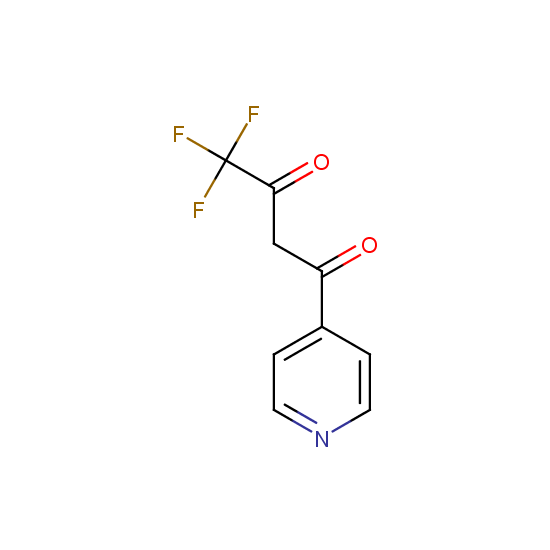
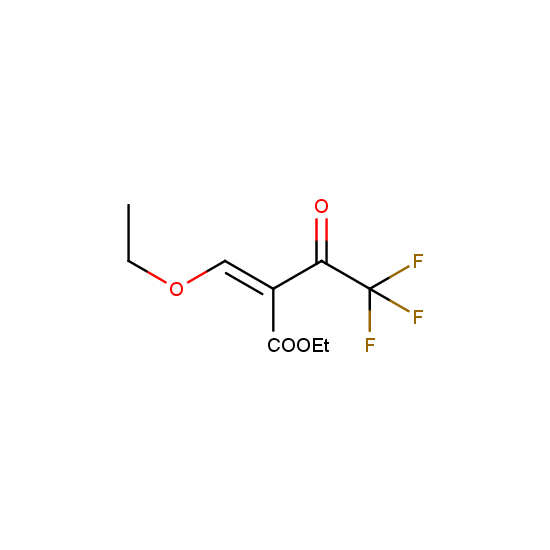
Trifluoromethyl β-dicarbonyl compounds:
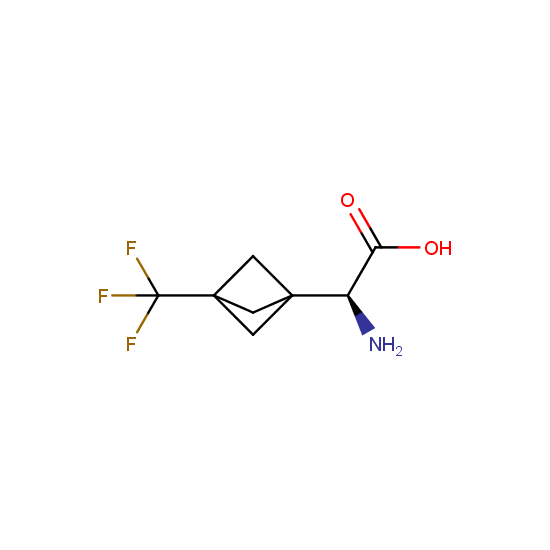
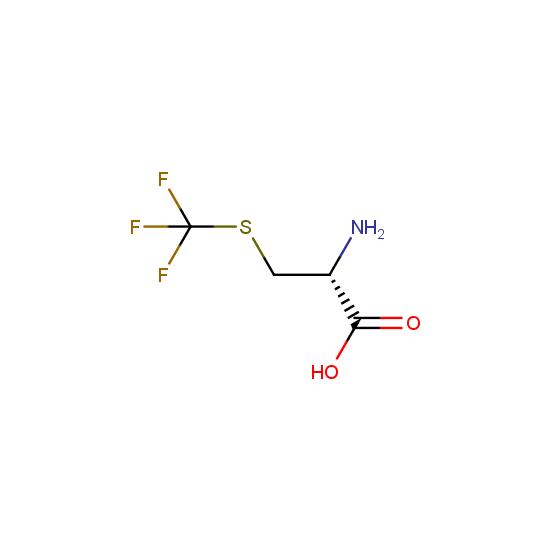
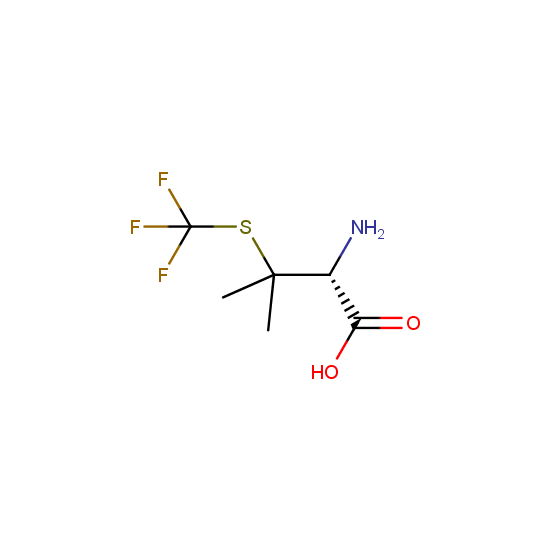
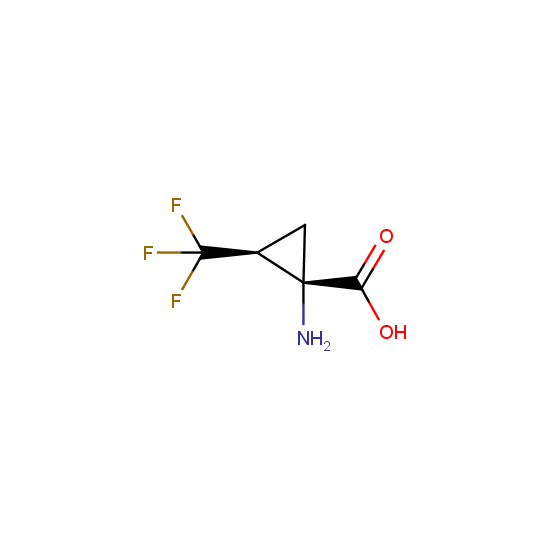
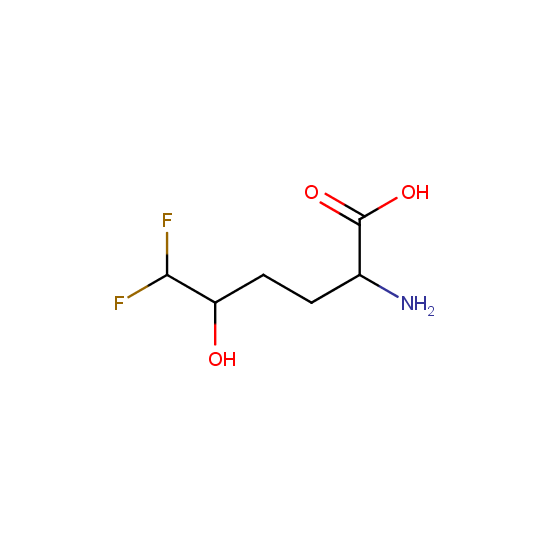
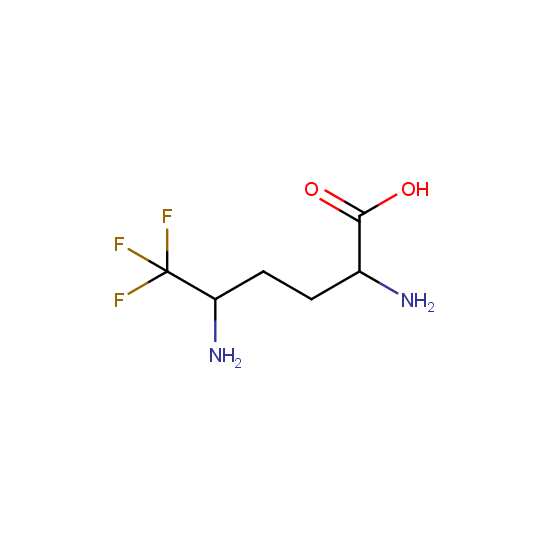
Amino acids:
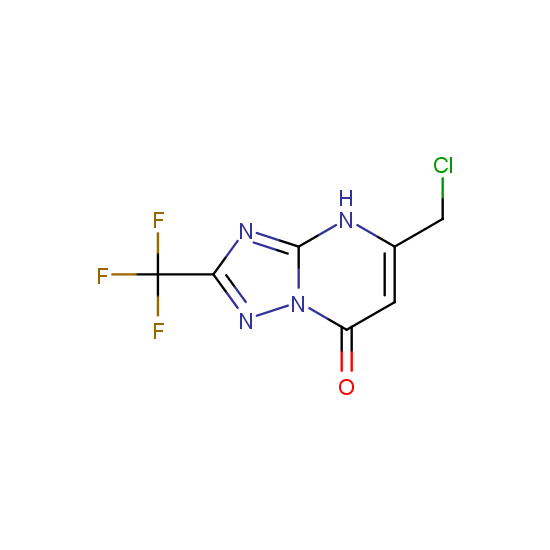
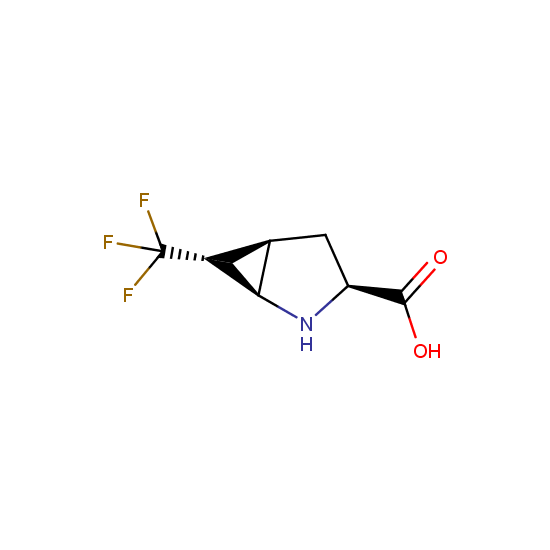
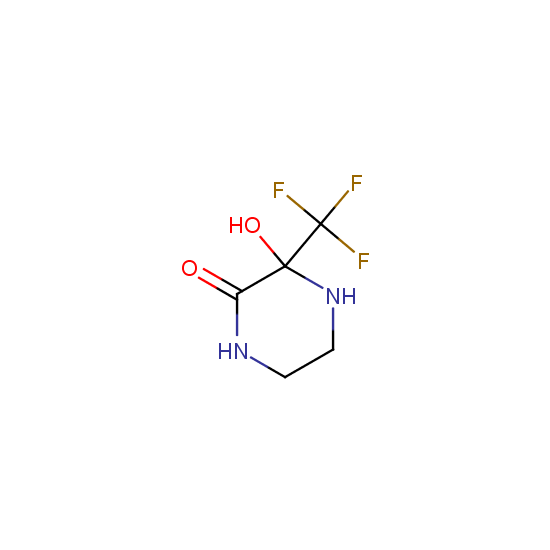
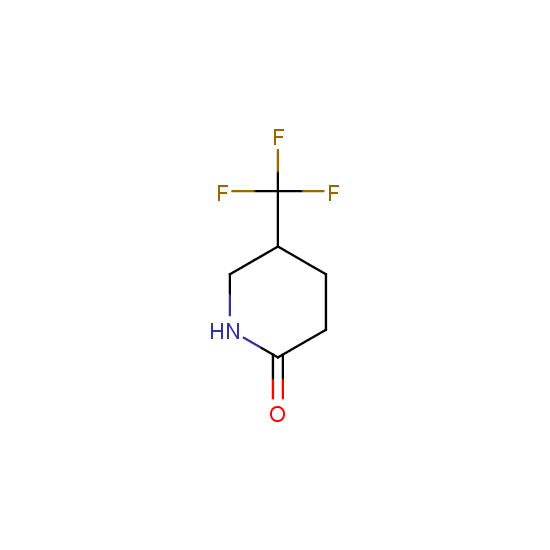
Cyclic amides: