The transition-metal-catalyzed cross-coupling reactions are powerful tools for the formation of carbon–carbon bonds. Among these transformations, the Suzuki–Miyaura reaction, which is the transition-metal-catalyzed cross-coupling between an organo-boron compound and an organic (pseudo)halide, has become the most attractive method since its discovery in 1979. While many organo-boron compounds have been discovered, N-methyliminodiacetic acid MIDA boronates represent tremendously useful building blocks for the Suzuki–Miyaura cross-coupling that has been successfully applied to sequential synthesis of various natural product motifs.
Read more: MIDA boronates: powerful tool for organic synthesis
Organic azides are an important class of synthetic building blocks. In organic chemistry azides are commonly used for introducing an amino-group via Staudinger reaction. With the recent advent of "click-chemistry" azides became enormously popular for their participation in the Cu(I)-catalyzed Huisgen azide-alkyne 1,3-dipolar cycloaddition reaction. Because of the high rate of synthetic success of both Huisgen cycloaddition and Staudinger reduction these reactions are often used in combinatorial and medicinal chemistry. Enamine presented the set of azides suitable for click-chemistry transformations earlier.
Fragment-baseajord drug design has become a novel paradigm for drug discovery in the last decade. The lack of molecular rigidity intrinsic to a majority of small organic molecules appears to be a serious obstacle for this promising approach. Conformationally restricted rigid molecules show higher reproducibility of results when used in projects utilizing in silico screening methods. Due to a predefined spatial orientation of the molecular fragments mounted on a conformationally rigid scaffold, a decrease in entropy of molecular binding with its biological target might be expected, thus leading to higher affinity of the potential drug candidate.
This issue of Enamine Product Focus highlights Lactams, cyclic amide building blocks. There are numerous examples of Lactams usage in drug discovery, e.g., β-lactam based antibiotics, oral anticoagulant Rivaroxaban, and anticonvulsant Levetiracetam.
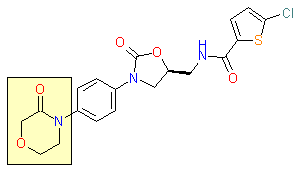
Rivaroxaban, 2008
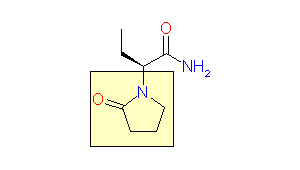
Levetiracetam, 2000
Bifunctional building blocks are of special interest for drug design and organic synthesis due to three reasons, at least. First, these compounds can be used to tether two molecular fragments responsible for binding to the biological target, thus they can act as linkers. Second, if one functional group is not engaged in connection between the core of building block and the rest of a molecule being constructed, then it can participate in important interaction with a biological target. Finally, many bifunctional building blocks can undergo cyclization reactions, allowing rapid advance toward prospective heterocyclic units.
