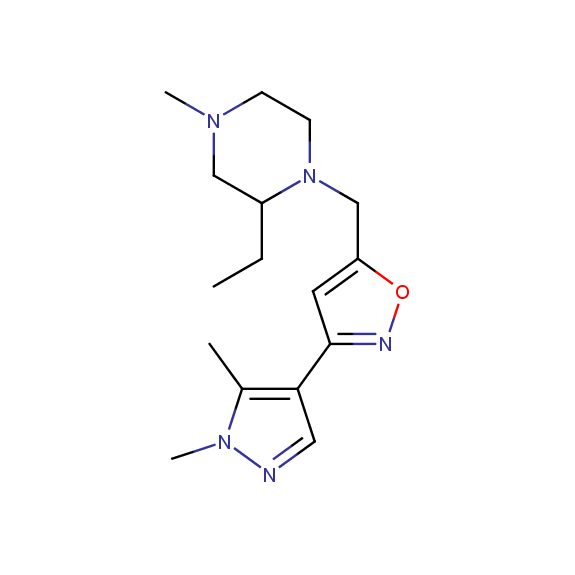
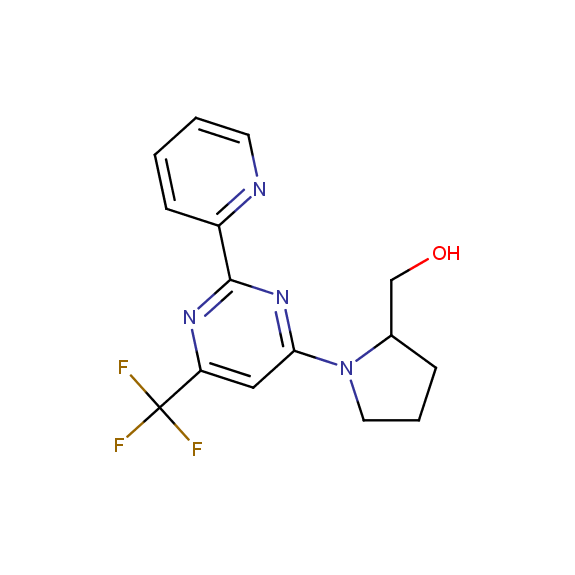
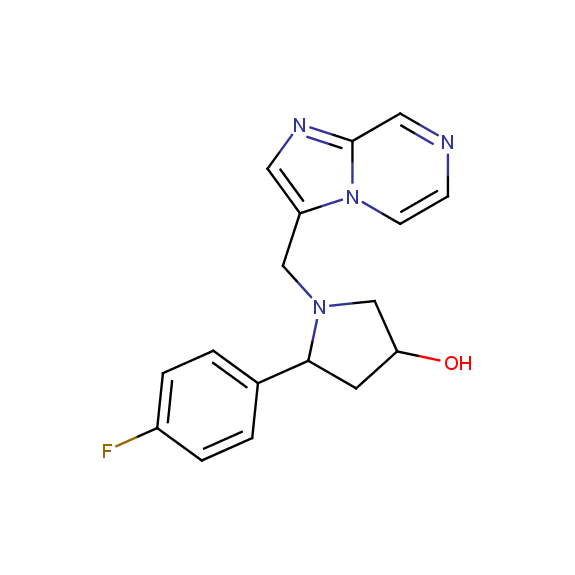
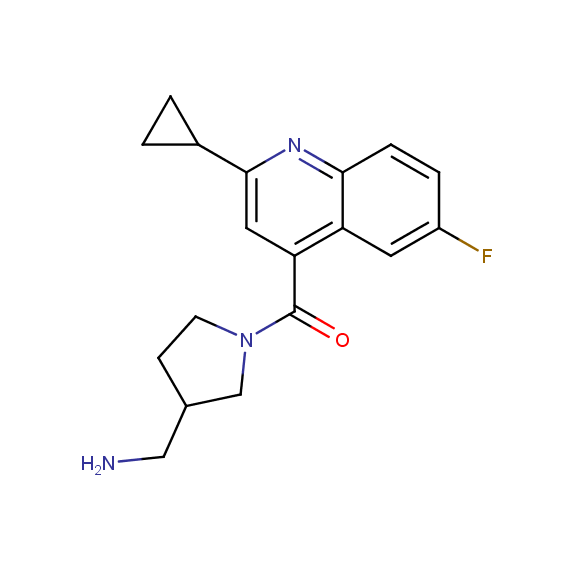
Designed for discovery of novel allosteric ligands
14 160 compounds
Although modulating GPCRs with orthosteric selective drug candidates has numerous success stories, in many cases, allosteric GPCR ligands are more effective for these biological targets. The small molecule allosteric modulation of GPCRs can promote a conformational change in the receptor that often has no noticeable effects, but can have a strong impact on the signaling pathway in the presence of an orthosteric endogenous ligand. On the other hand, GPCRs actuated by intractable stimuli can be modulated allosterically, opening a new opportunity for these currently unassailable targets. Two allosteric GPCRs modulators that have been introduced in the clinic (Cinacalcet and Maraviroc) are excellent evidence of prospects of investigation in this area. Another benefit of these ligands is their relatively low molecular weight and hence higher potential for oral bioavailability, in contrast to most orthosteric ligands. Moreover, allosteric sites are generally less conserved enabling development of actives with greater subtype selectivity than drugs targeting the conserved binding site.
Enamine’s Allosteric GPCR Library is a utile starting point for the drug discovery in the field of allosteric GPCR modulators.
Typical Formats
Allosteric GPCR Library is available for supply in various pre-plated formats, including the following most popular ones:
Catalog No.
AGR-14-0-Z-10
Compounds
14 160
12 plates
Amount
≤ 300 nL of 10 mM of DMSO solutions
Plates and formats
1536-well Echo LDV microplates, first and last four columns empty, 1280 compounds per plate
Price
Catalog No.
AGR-14-10-Y-10
Compounds
14 160
45 plates
Amount
≤ 10 µL of 10 mM DMSO solutions
Plates and formats
384-well, Echo Qualified LDV microplates #001-12782 (LP-0200), first and last two columns empty, 320 compounds per plate
Price
Catalog No.
AGR-14-50-Y-10
Compounds
14 160
45 plates
Amount
50 μL of 10 mM DMSO solutions
Plates and formats
384-well, Greiner Bio-One plates #781280, 1,2 and 23,24 columns empty, 320 compounds per plate
Price
Catalog No.
Library & follow-up package
Plates and formats
AGR-14-10-Y-10 screening library 14 160 cmpds, hit resupply, analogs from 4.7M+ stock and synthesis from REAL Space
Price
*We will be happy to provide our library in any other most convenient for your project format. Please select among the following our standard microplates: Greiner Bio-One 781270, 784201, 781280, 651201 or Echo Qualified 001-12782 (LP-0200), 001-14555 (PP-0200), 001-6969 (LP-0400), C52621 or send your preferred labware. Compounds pooling can be provided upon request.
Download SD files
Library code: AGR-14
Version: 2 September 2024
14 160 compounds
sublibrary of GPR-54
Library design
Both ligand- and structure-based methods were used in design of the library. Ligand-based approach relied on using 3D pharmacophore search to the reference set of known ligands with high activity values (over 200 compounds from ChEMBL and BindingDB). The main emphasis has been done on allosteric modulators of class B GPCRs that are well established in drug discovery. Two pharmacophore models were created using the reference compounds sets and then validated with the training sets of actives and non-active ligands (Figure 1). Finally, over 6 200 small molecules were selected into Pharmacophore-based allosteric GPCR subset starting from Enamine MedChem set (710 K).
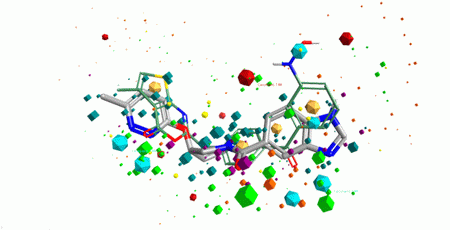
Fig. 1. Pharmacophore superposition of a reference active compound (in green) and a hit compound from the Library (in grey).
Structure-based approach was applied to human Glucagon receptor (GCGR) and human Corticotropin-releasing factor 1 receptor (CRF1R). The corresponding protein structures recorded in 5EE7 and 4K5Y PDB entries were optimized and used for the docking calculation with Glide (Schrödinger software). Compounds with at least 50% score-based efficacy as compared to the co-crystallized ligands were selected as hits and included in the targeted sets: h-GCGR set – 200 compounds; h-CRF1R set: 7 200 compounds (Figures 2 and 3).
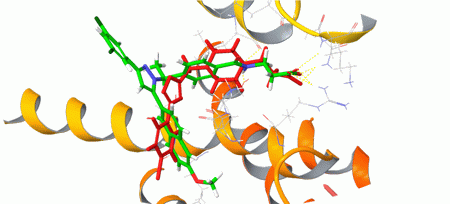
Fig. 2. Binding mode of a hit (in red) obtained from the docking and the co-crystallized ligand (in green) of human Glucagon receptor.
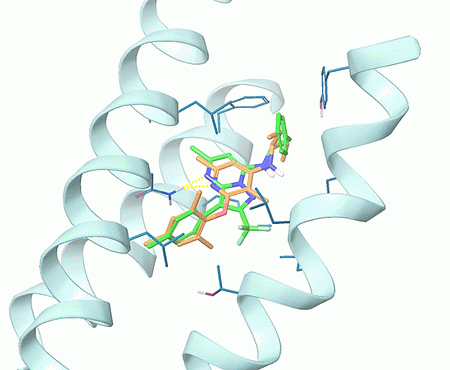
Fig. 3. Superposition of a hit compound obtained after docking of CRF1R allosteric site with Enamine MedChem screening compounds (in green) and the co-crystallized ligand CP-376395 (in orange).