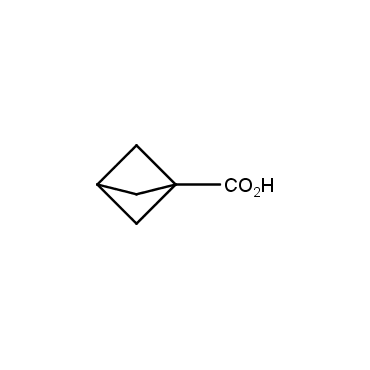
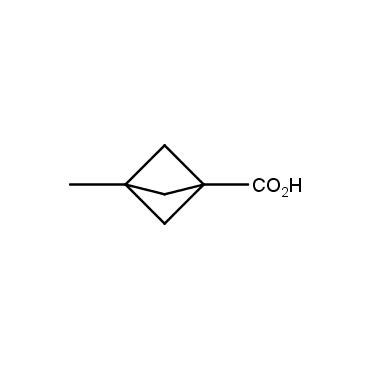
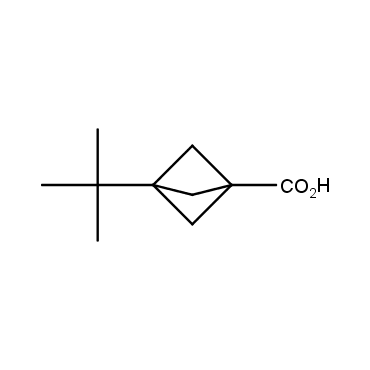
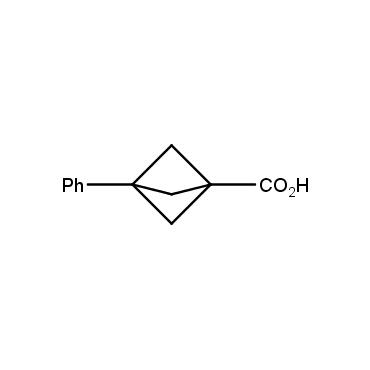
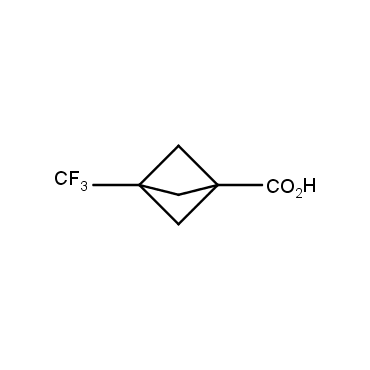
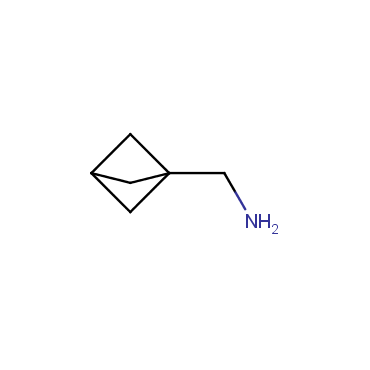
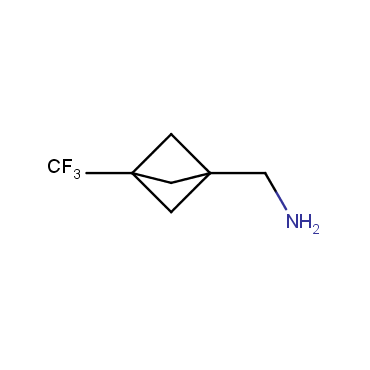
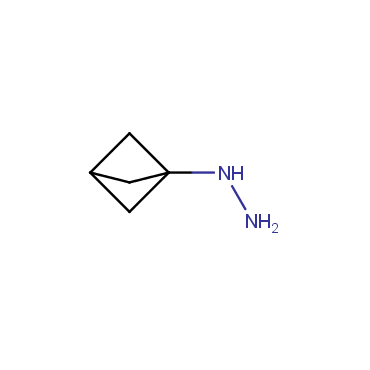
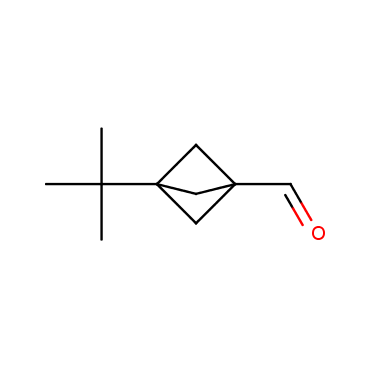
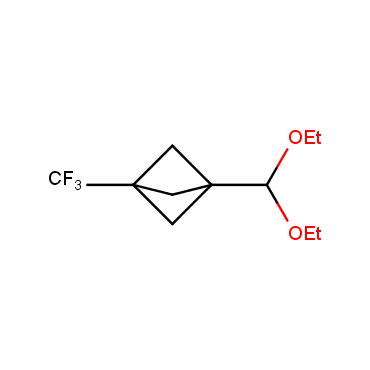
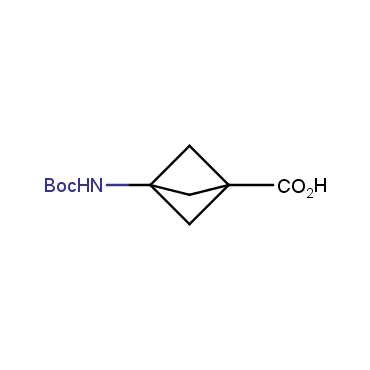
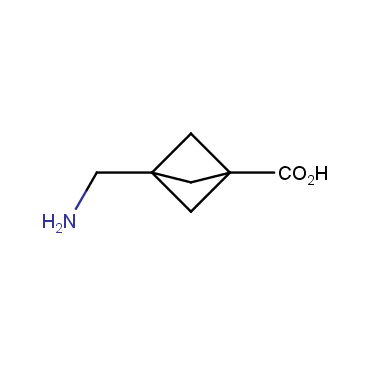
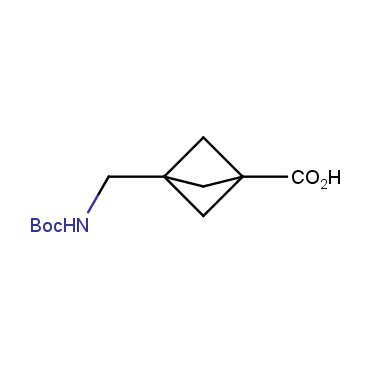
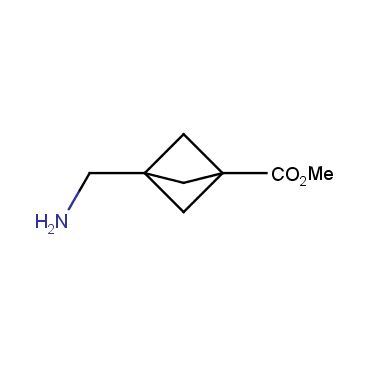
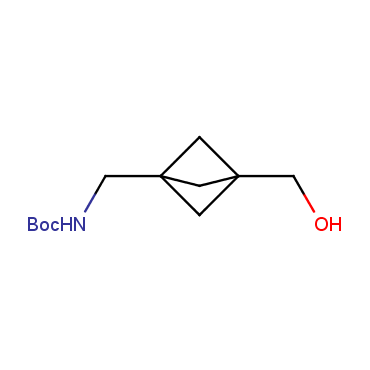
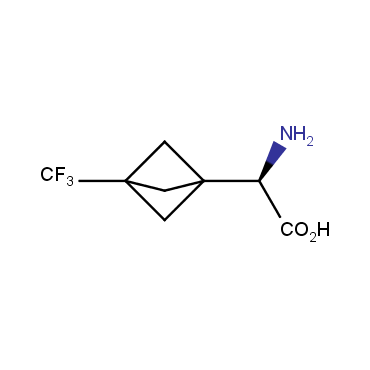
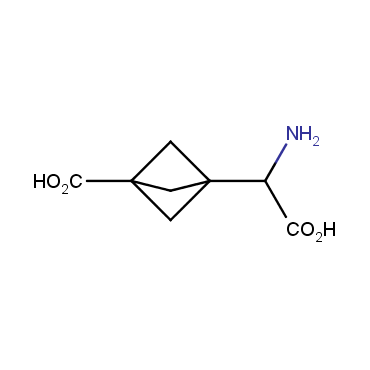
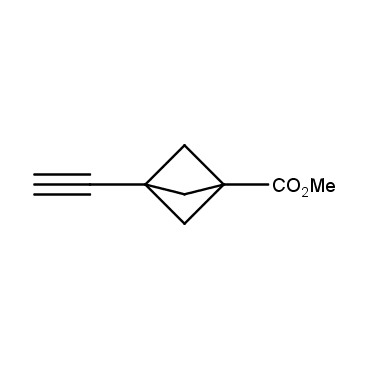
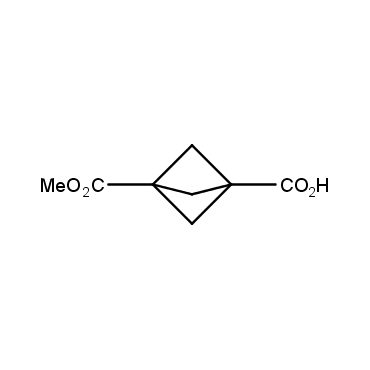
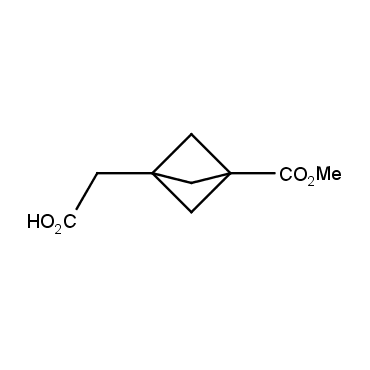
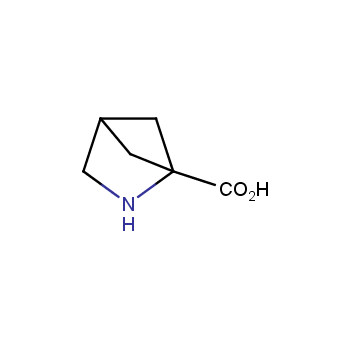
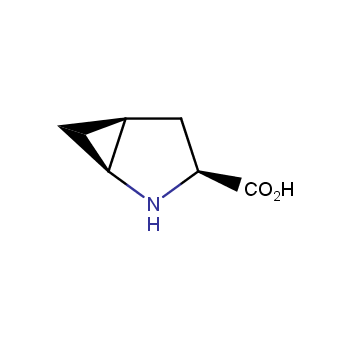
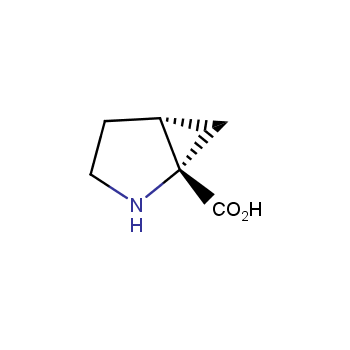
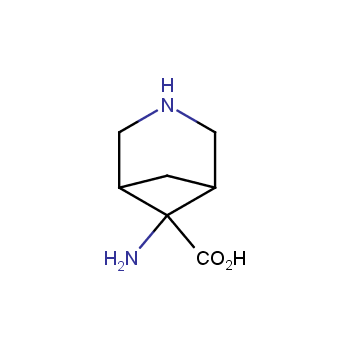
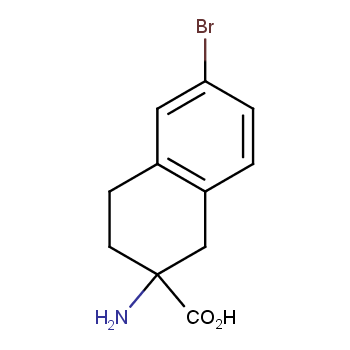
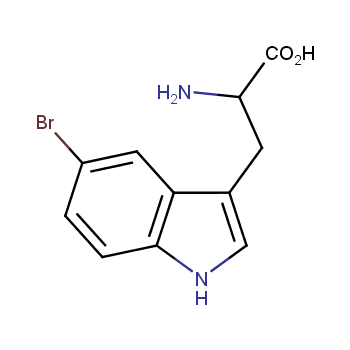
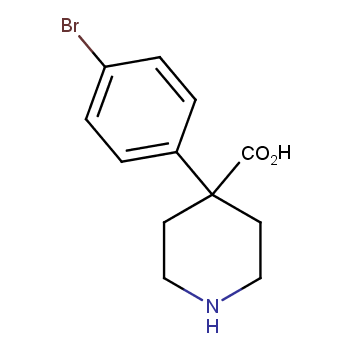
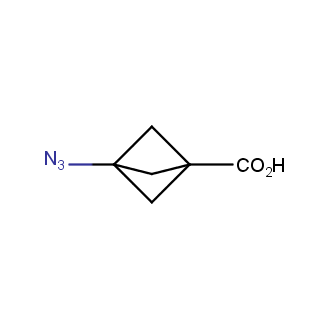
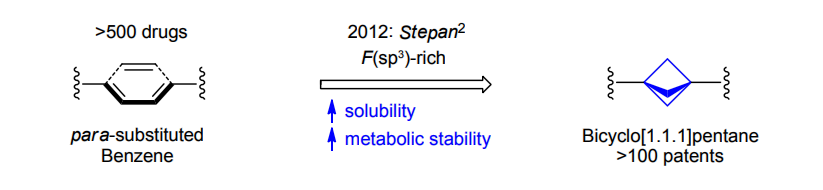
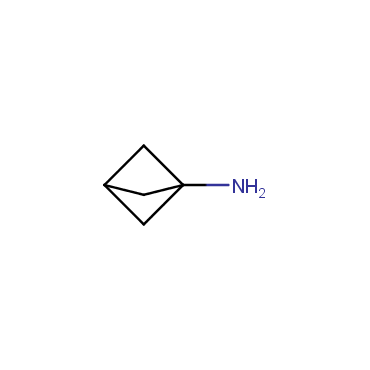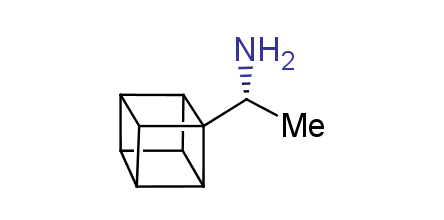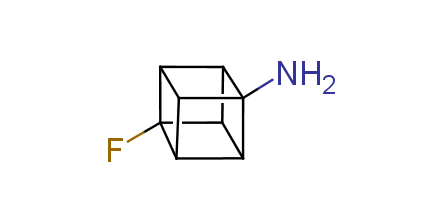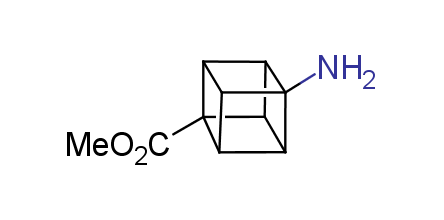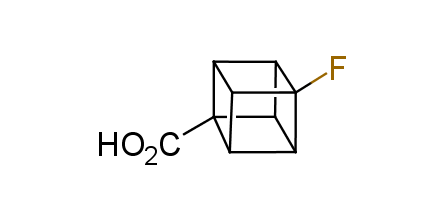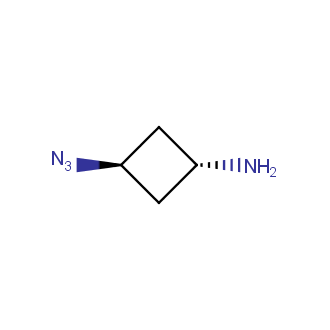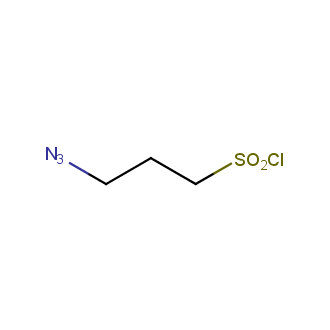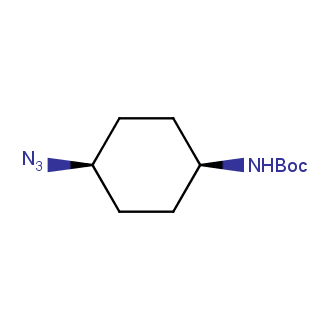The residue of benzene comprises to the structure of more than 500 FDA-approved drugs. In 2012, Stepan and coworkers showed that bicyclo[1.1.1]pentane skeleton could act as a saturated "nonclassical phenyl ring bioisostere" in the design of a γ-secretase inhibitor. Since then, the core of bicyclo[1.1.1]pentane is often used in the design of analogues of natural compounds, peptide studies, medicinal chemistry, and supramolecular chemistry. Herein we have designed and synthesized a library of saturated mimics of the para-benzene ring for drug design.

Design
Download SD file
Download PDF file
We offer
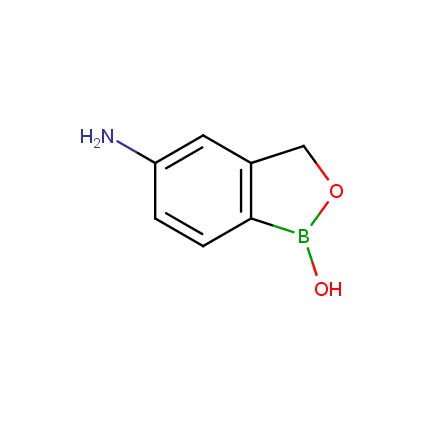
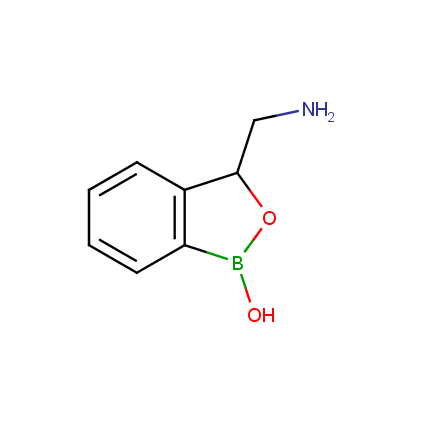
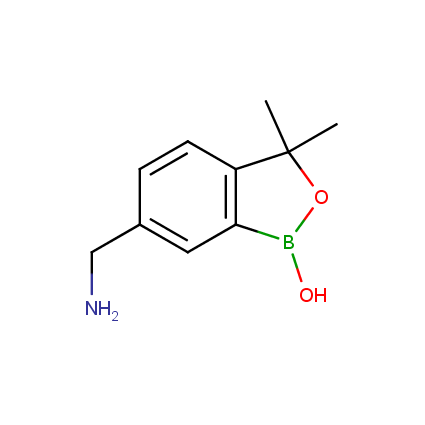
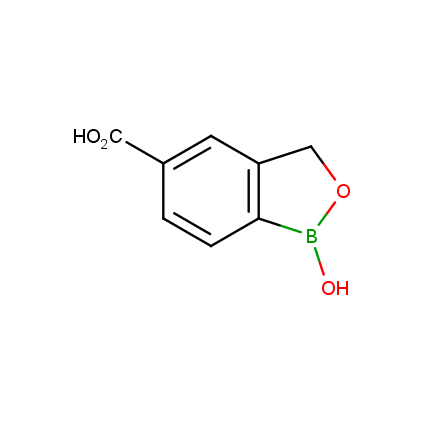
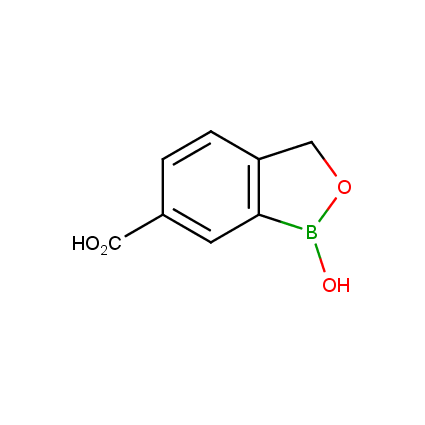
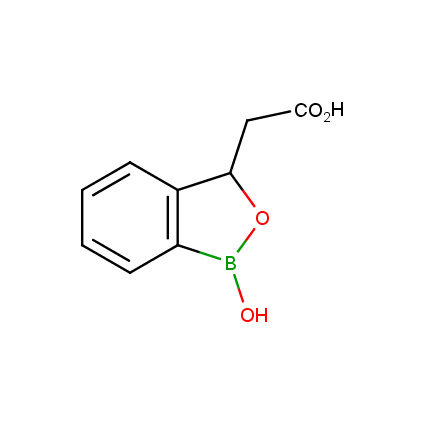
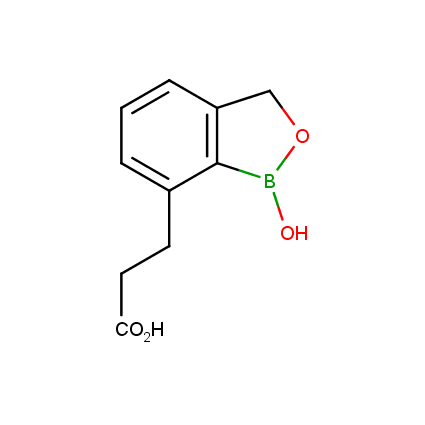
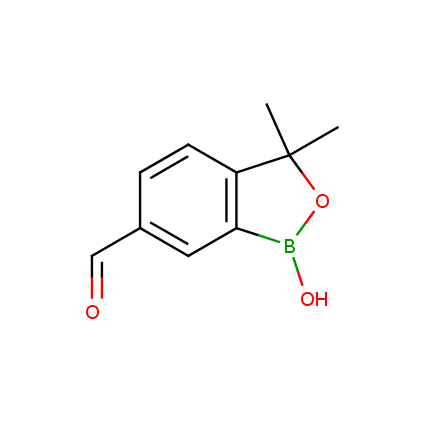
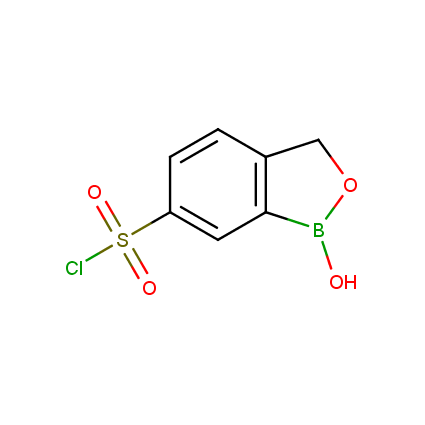
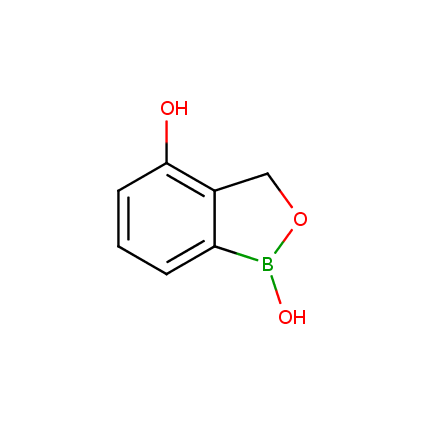
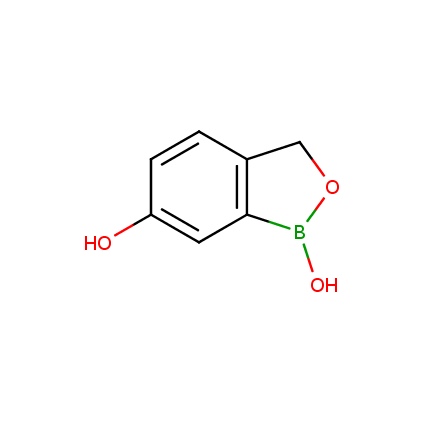
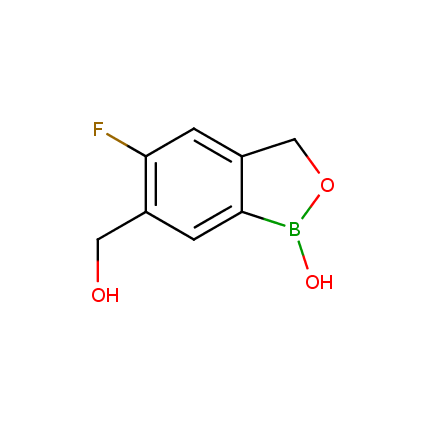
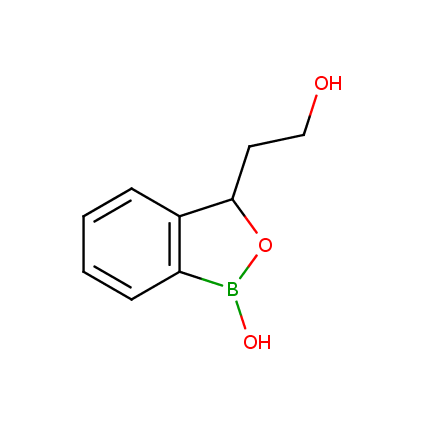
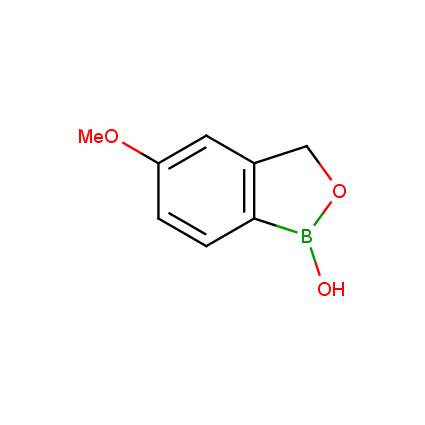
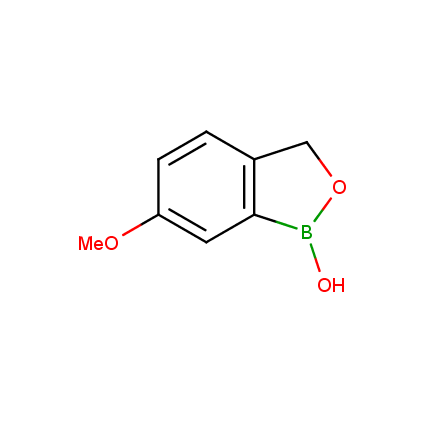
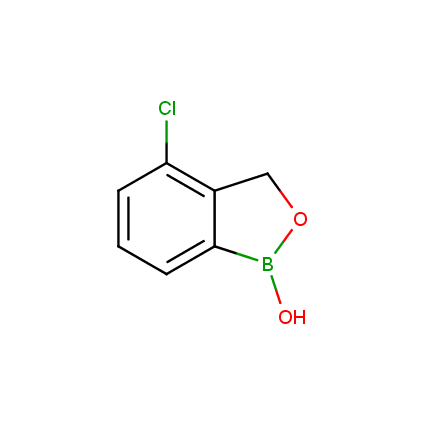
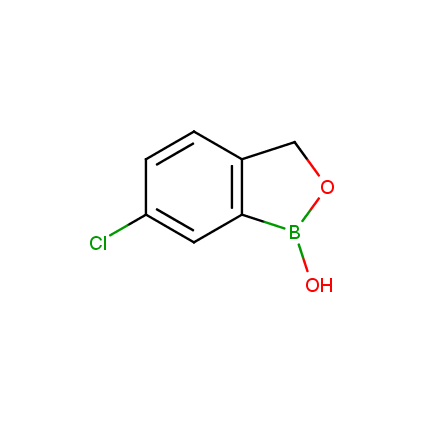
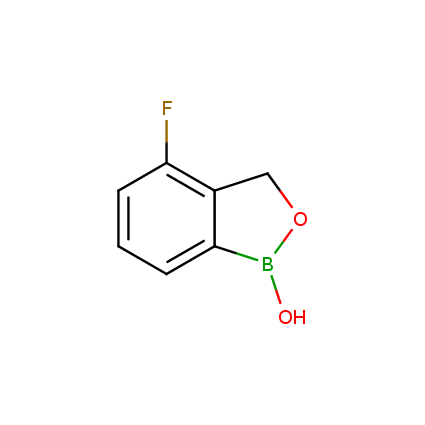
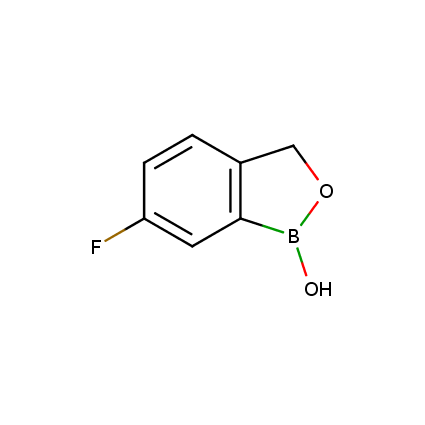
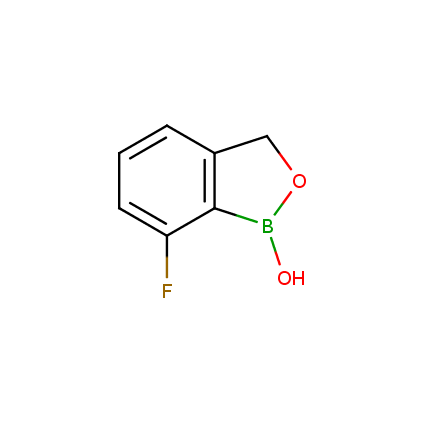
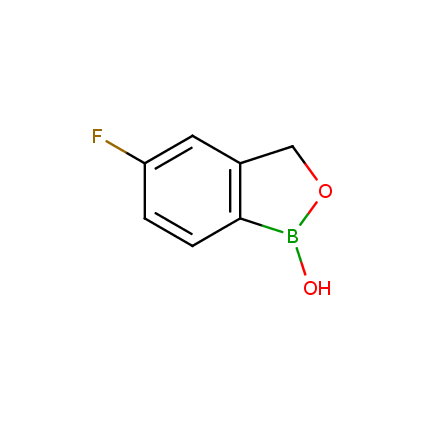
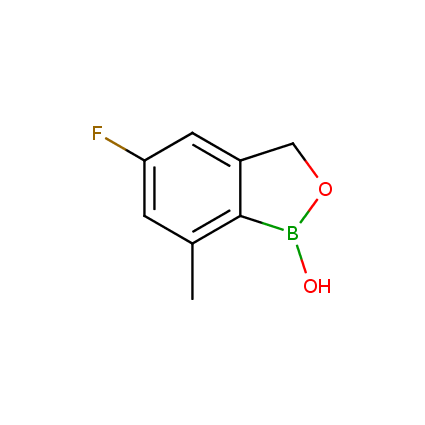
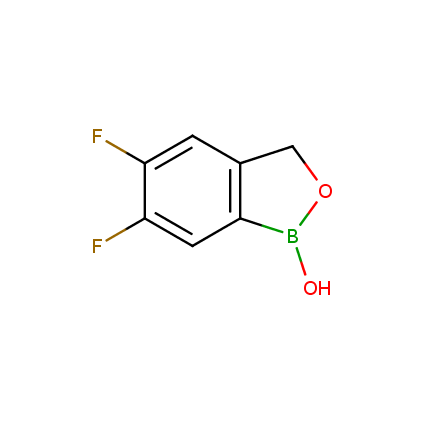
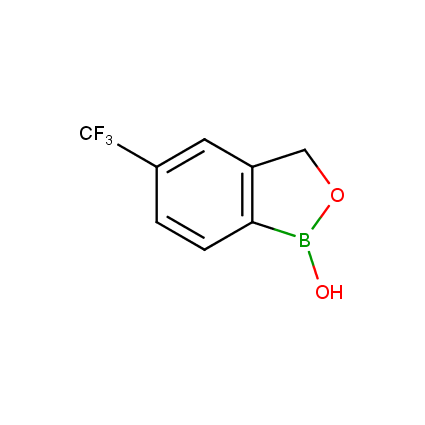
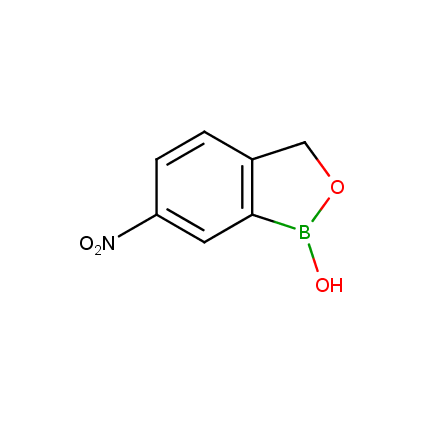
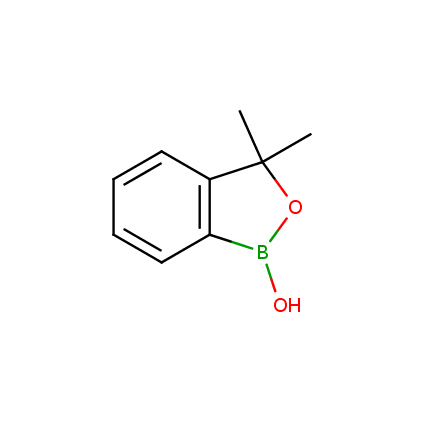
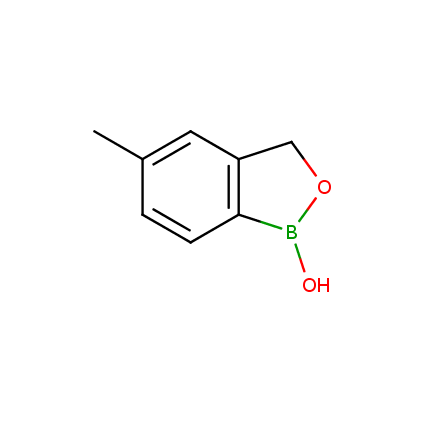
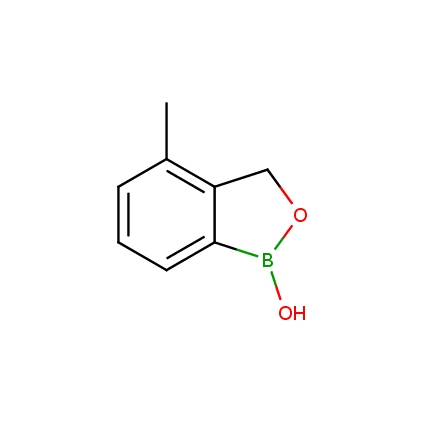
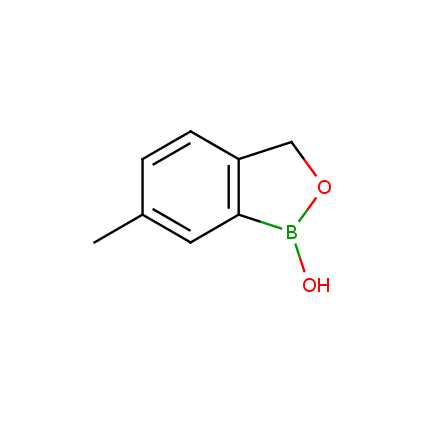
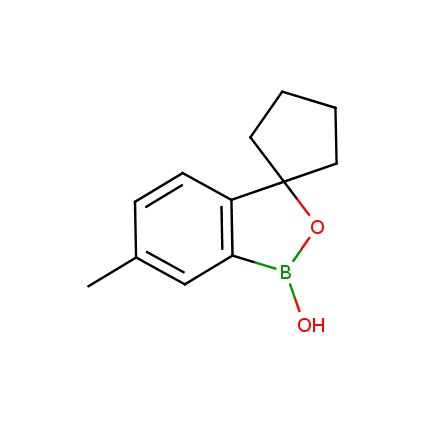
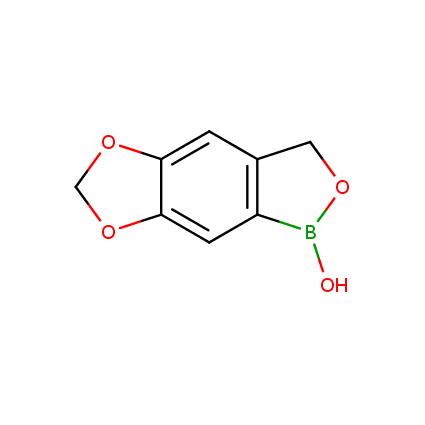
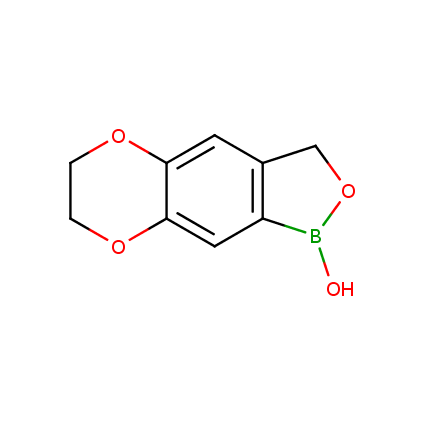
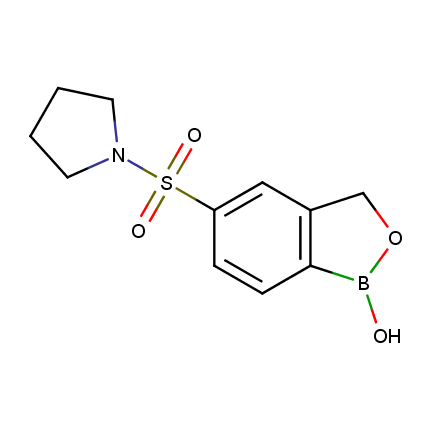
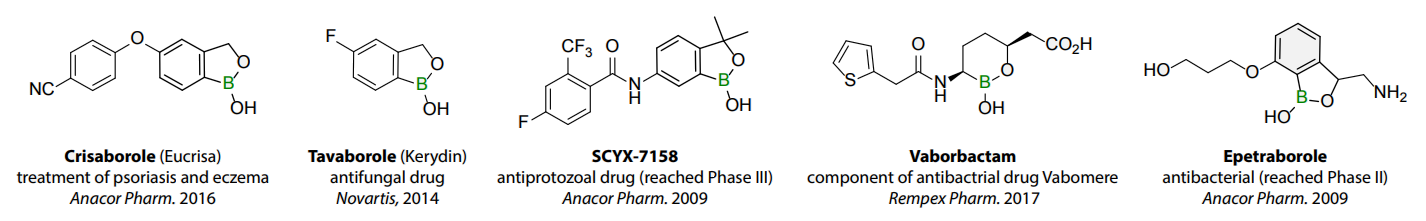
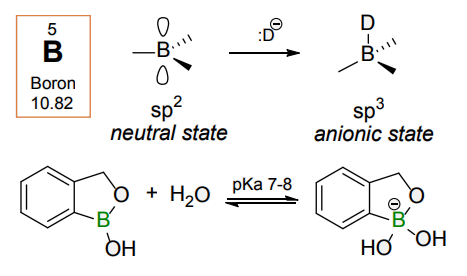
Benzoxaborole is a versatile boron-heterocyclic scaffold which has found in the last 10 years a broad spectrum of applications in medicinal chemistry. The use of benzoxaborole moiety in the design of compounds led to the discovery of new classes of anti-bacterial, anti-fungal, anti-protozoal, anti-viral and as anti-inflammatory agents with interesting drug development perspectives. Two benzoxaborole derivatives are already clinically used for the treatment of onychomycosis (Tavaborole) and atopic dermatitis (Crisaborole), with several others in various phases of clinical trials.
Advantages
Properties of benzoxaboroles:
- Low biotoxicity.
- Good solubility in water.
- They can coordinate to O and N.
- Ability to interfere protein synthesis.
- Covalent bonding- and nonbonding interactions with protein targets.
We offer
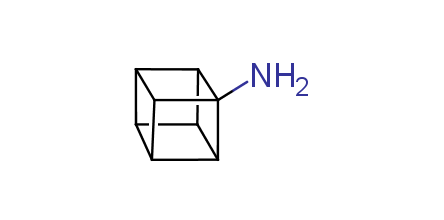
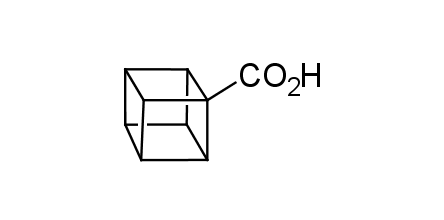
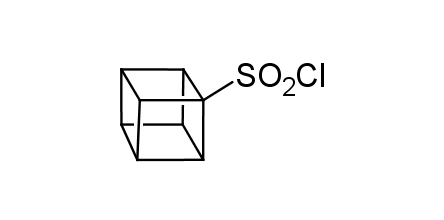
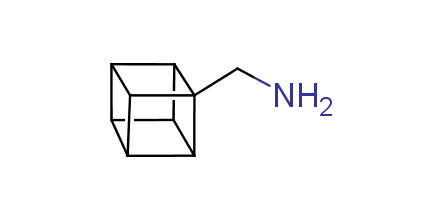
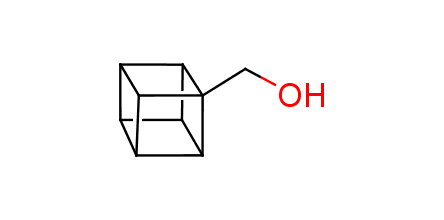
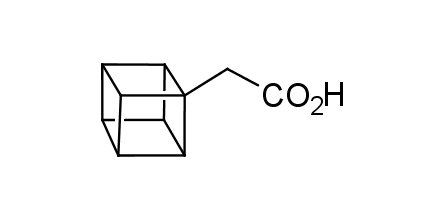
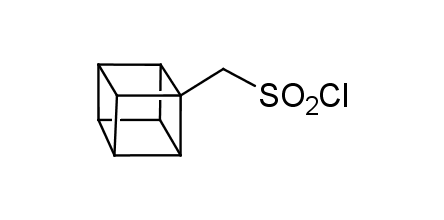
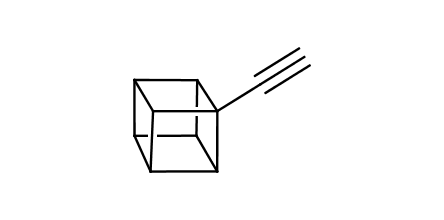
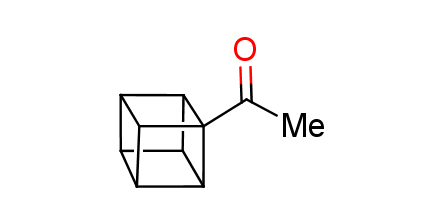
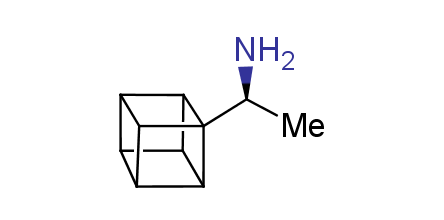
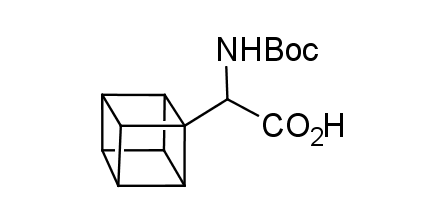
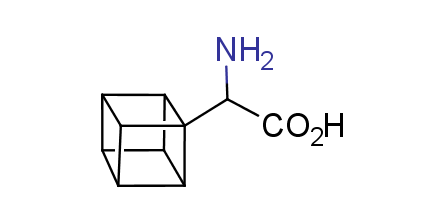
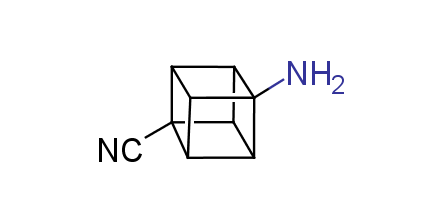
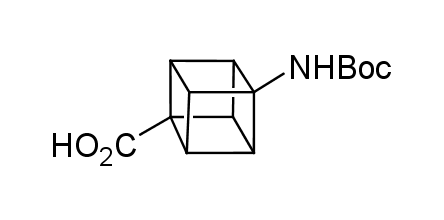
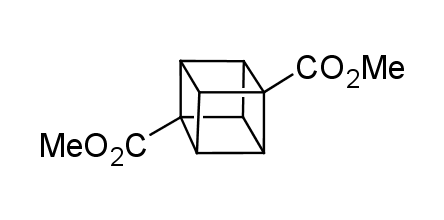
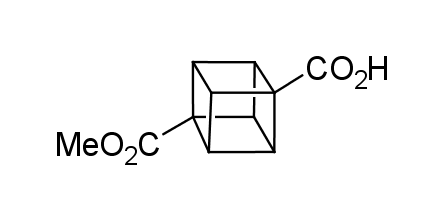
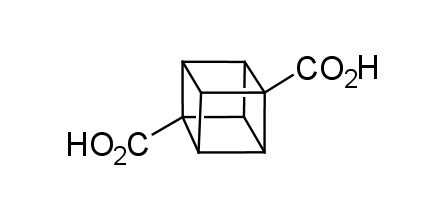
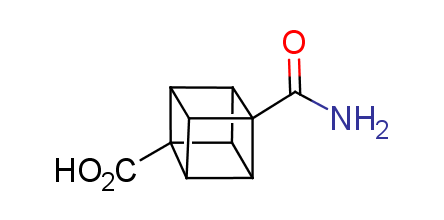
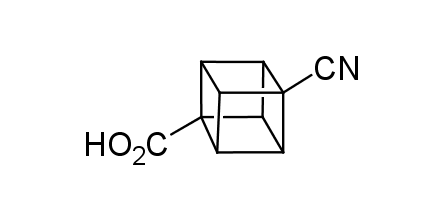
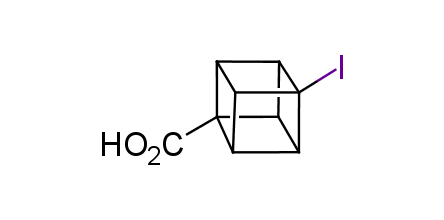
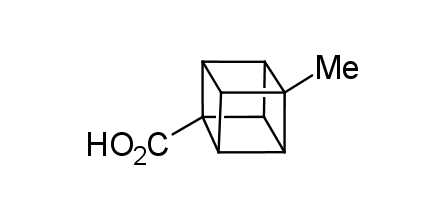
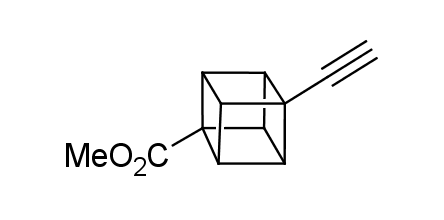
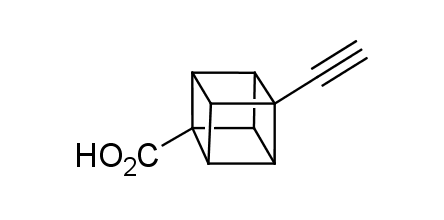
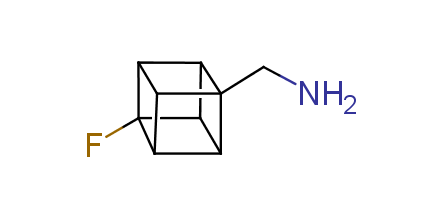
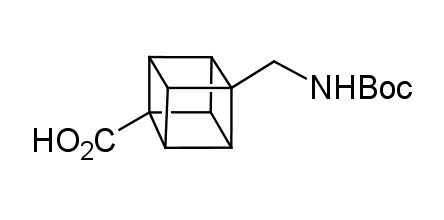
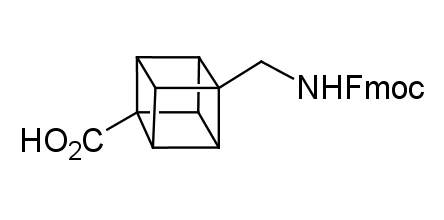
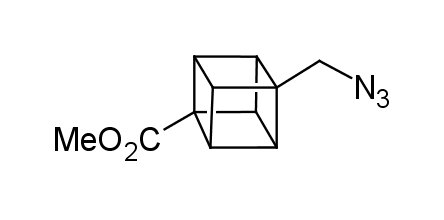
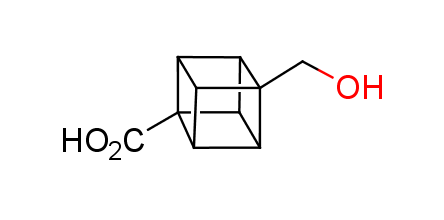
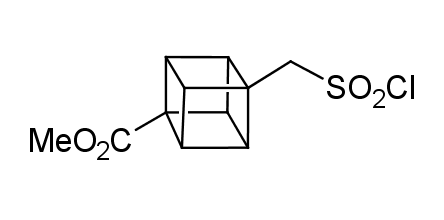
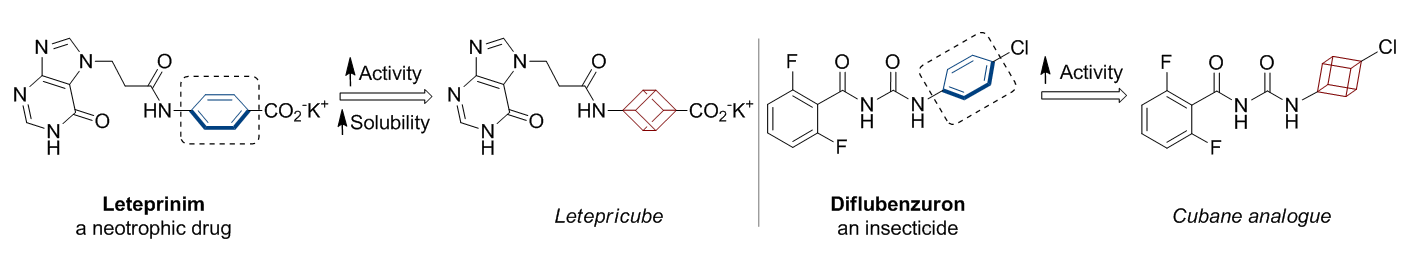
In 1992, Eaton predicted a high potential of cubane in a pharmaceutical research as a bioisoster of benzene, based on their similarity in size. In 2016, Williams and collaborators showed that replacing a benzene ring in the neurotropic compound Leteprinim with cubane beneficially affected activity and solubility of the parent compound. The cubane analogue significantly outperformed pesticide Diflubenzuron. Since then the cubane-containing building blocks have been playing an important role in medchem projects, as mimics for the para-substituted benzene ring. In this context, Enamine offers a library of cubane-containing building blocks for drug design.
Concept
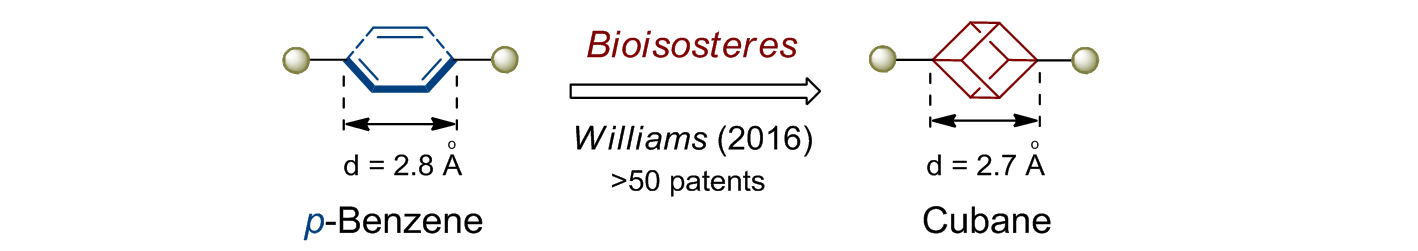
We offer:
Cubane-containing building blocks from stock on a 5-10 g scale.
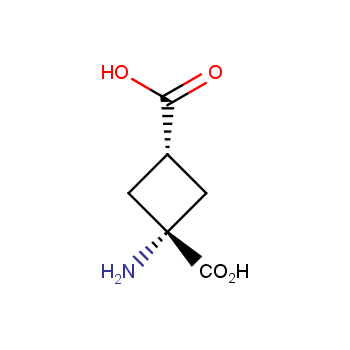
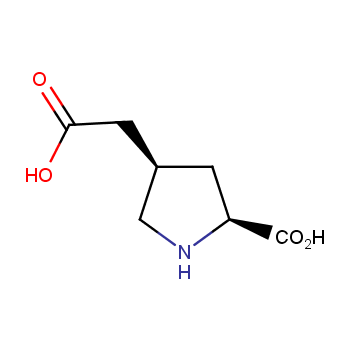
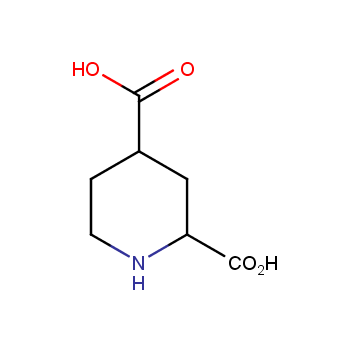
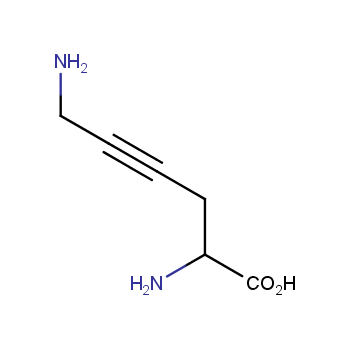
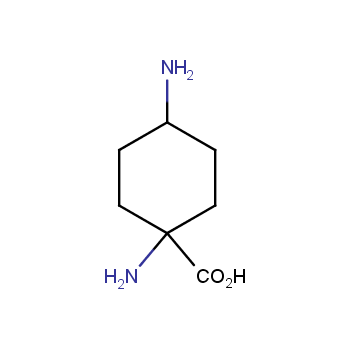
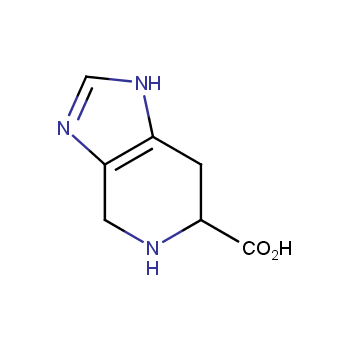
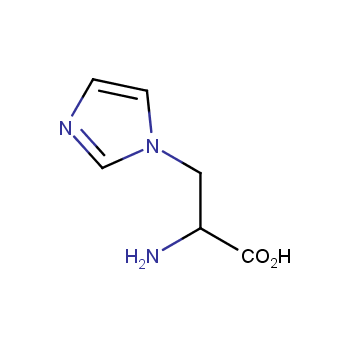
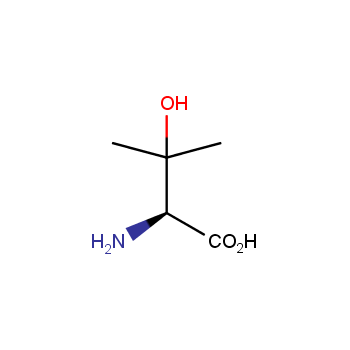
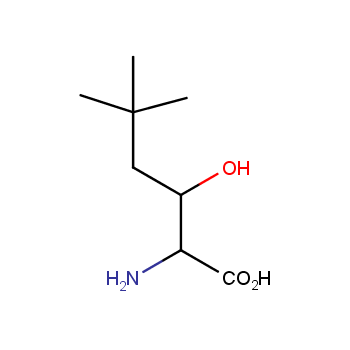
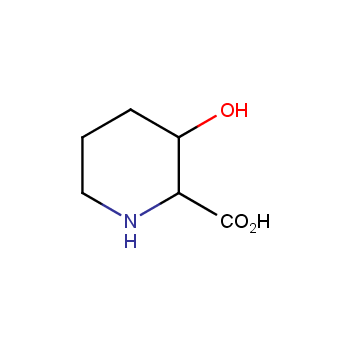
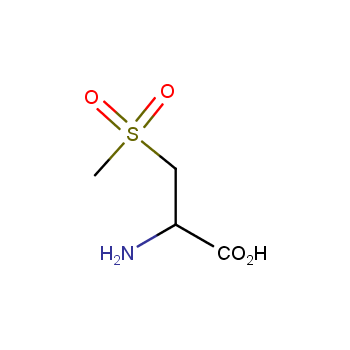
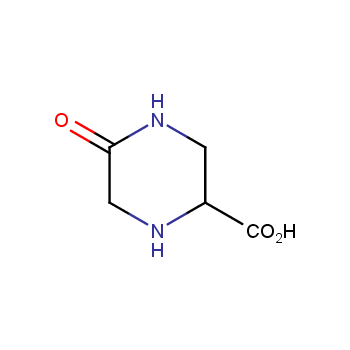
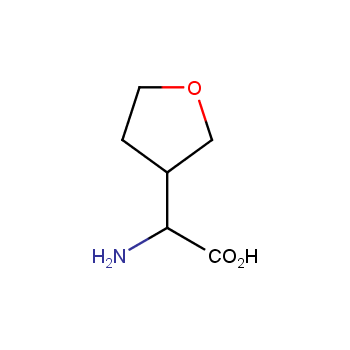
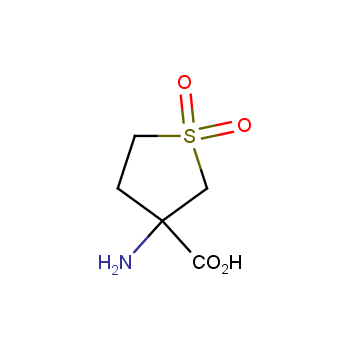
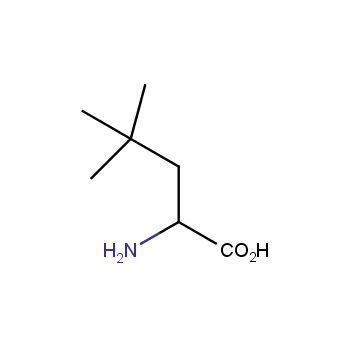
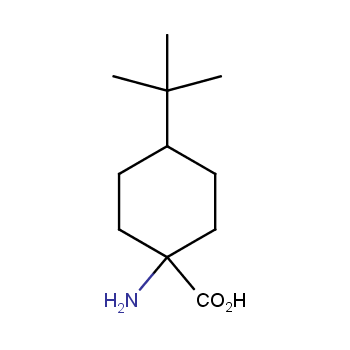
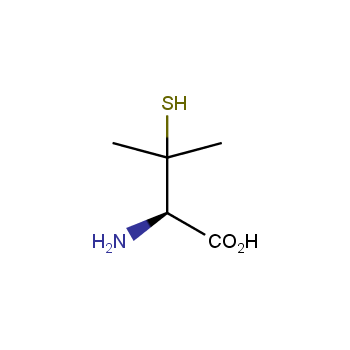
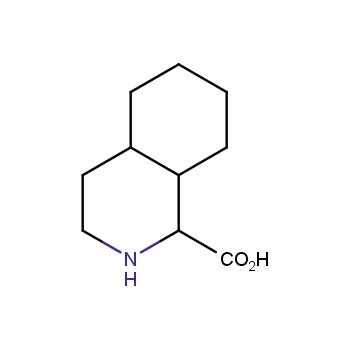
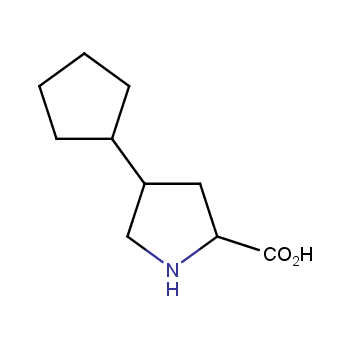
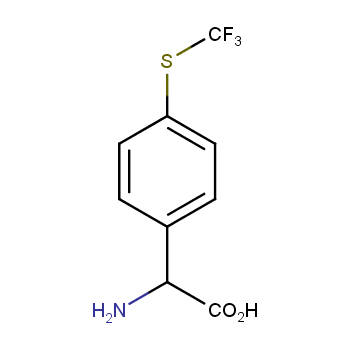
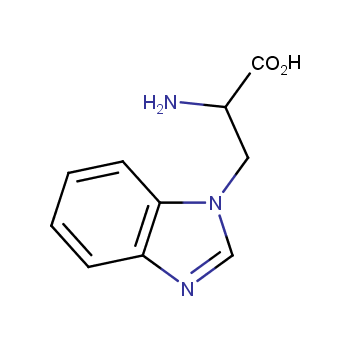
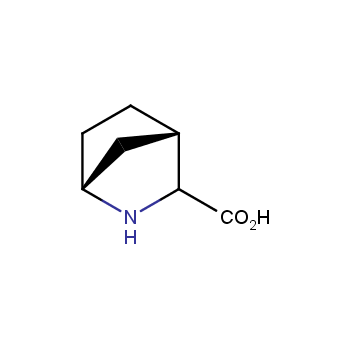
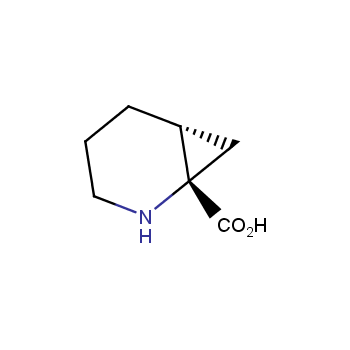
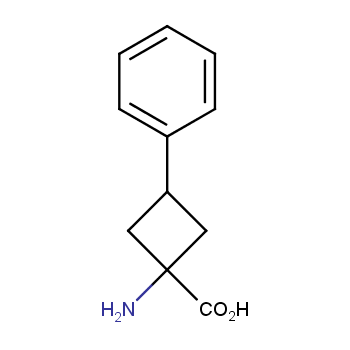
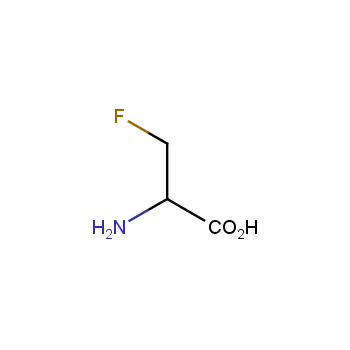
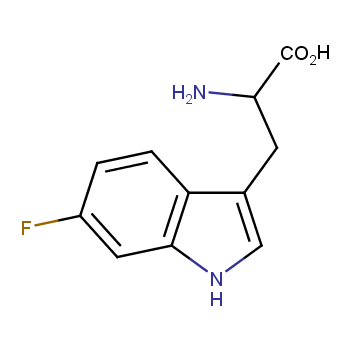
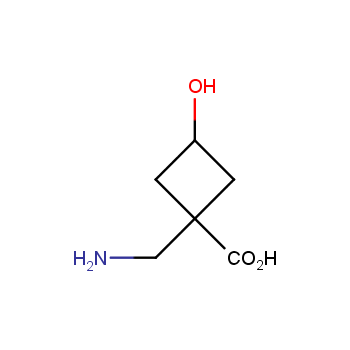
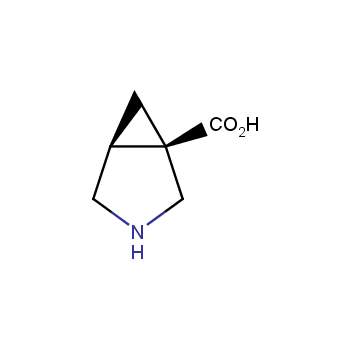
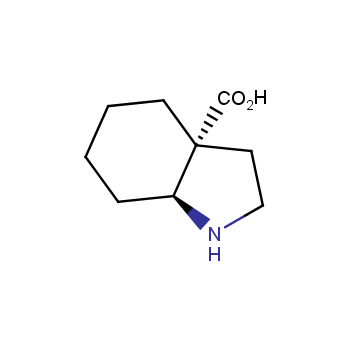
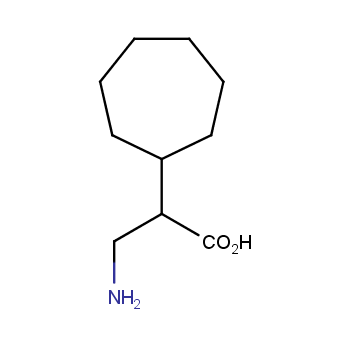
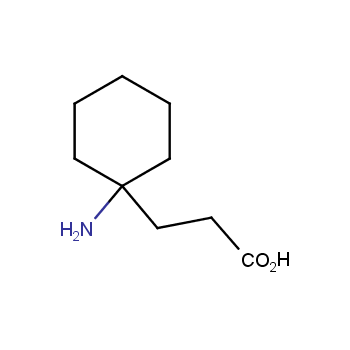
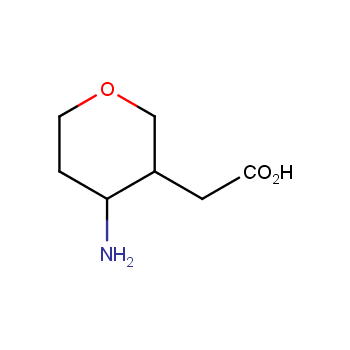
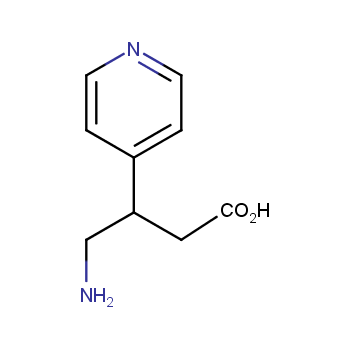
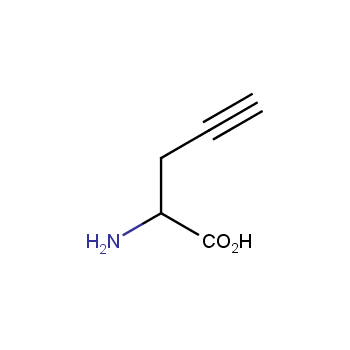
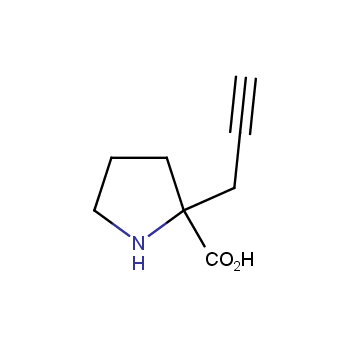
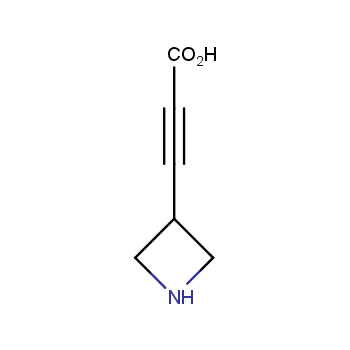
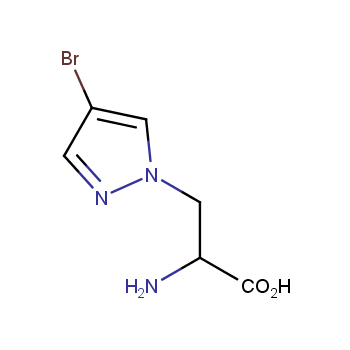
Amino acids are a privileged class of building blocks in drug design. Synthesis of new unusual amino acids has always been in focus of Enamine’s research since its foundation 26 years ago. We are proud to offer the world’s largest collection of unnatural amino acids from our stock and offer our skills and expertise in synthesis of custom compounds or compound libraries.
Our offer:
- Over 3 000 amino acids in stock on a gram scale, racemic mixtures and pure enantiomers
- Over 1 000 000 synthetically accessible REAL amino acids, lead time 5-6 weeks, feasibility 75%
- Focus on DNA-encoded library synthesis: supply of Fmoc-derivatives in µmol amounts
- Custom synthesis of amino acids, their derivatives, and compound libraries
Amino acids with charged side chains
Amino acids with polar and hydrophilic side chains
Amino acids with hydrophobic side chains
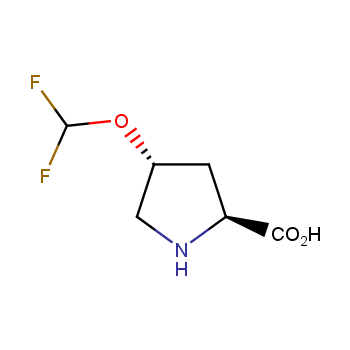
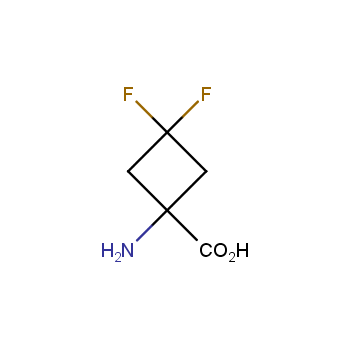
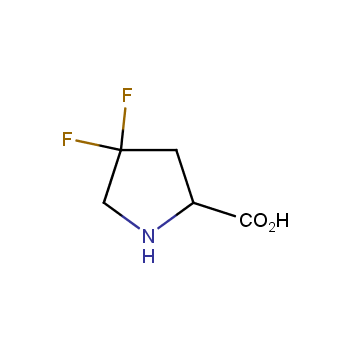
Conformationally restricted amino acids
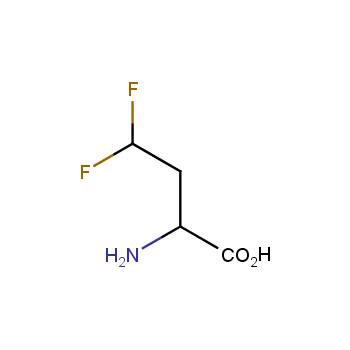
Fluorinated amino acids
β−and γ−Amino acids
Polyfunctional, DEL-compatible amino acids
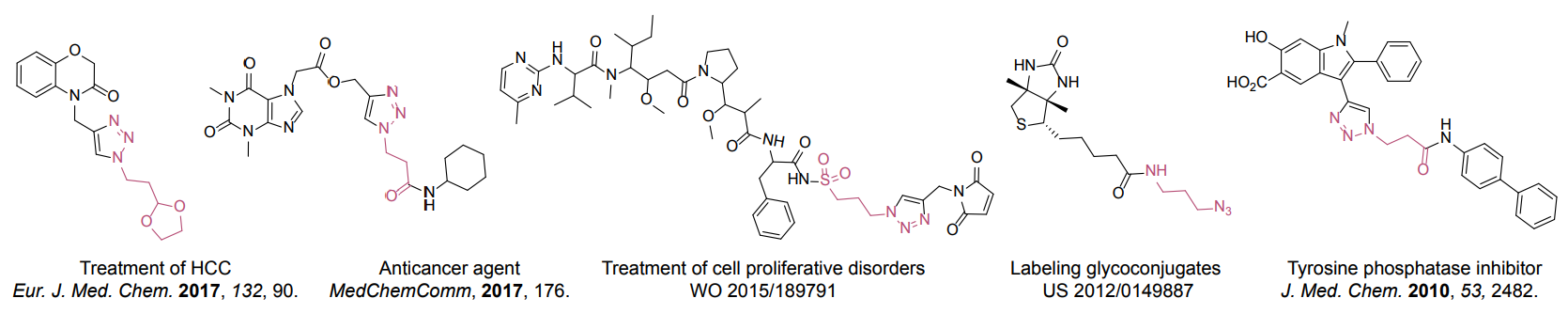
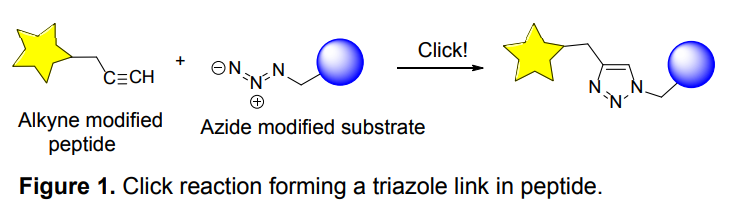
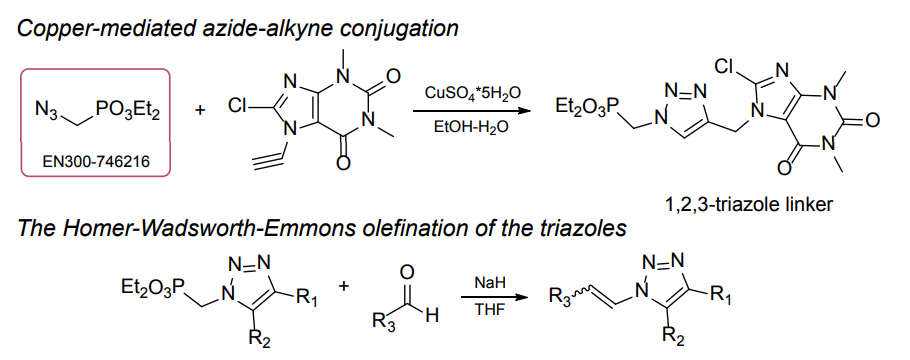
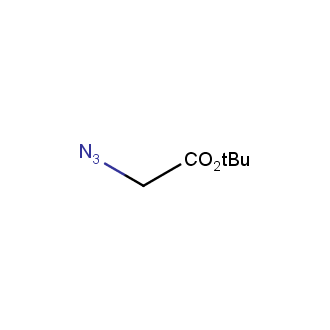
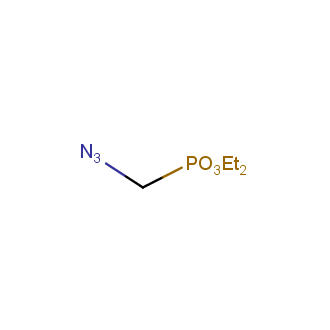
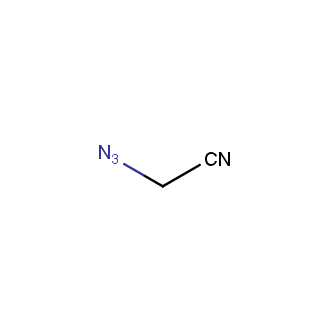
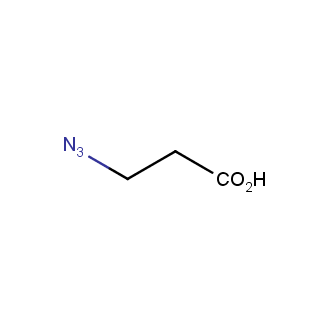
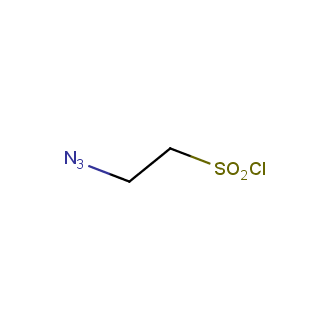
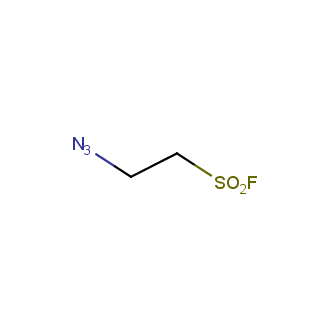
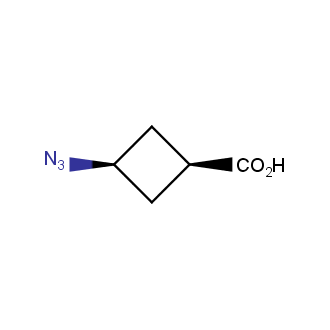
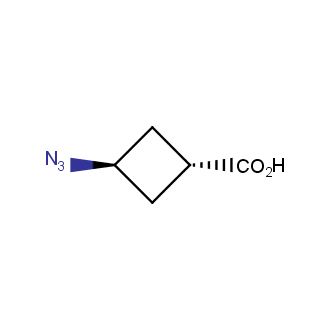
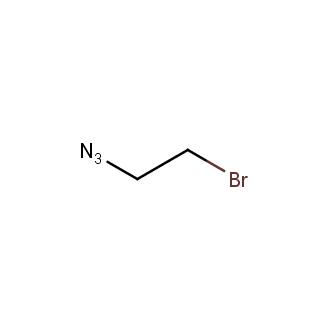
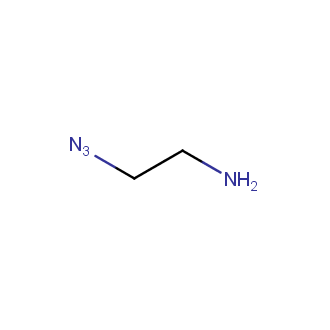
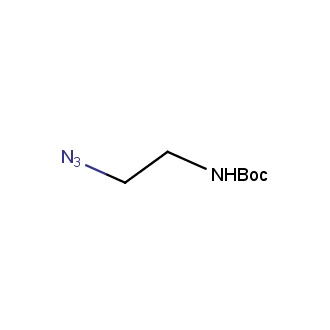
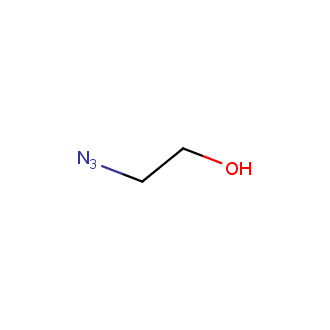
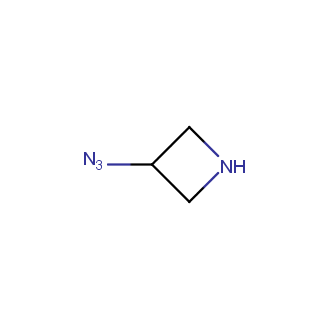
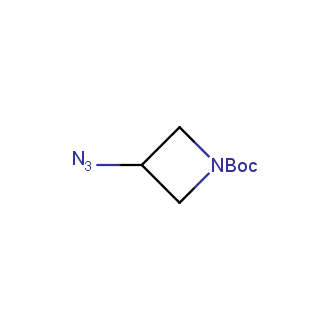
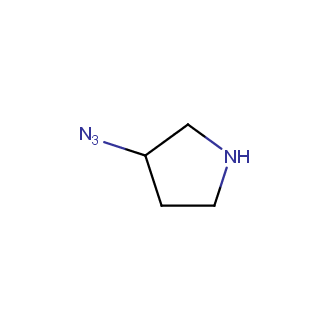
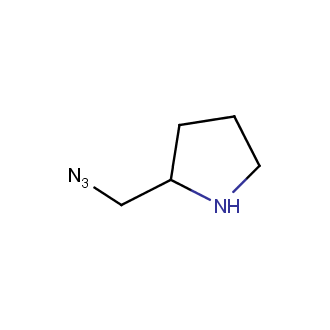
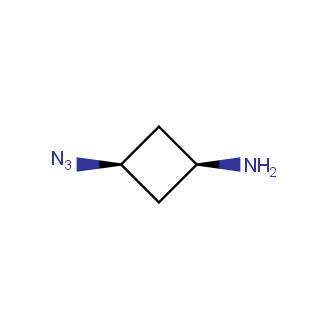
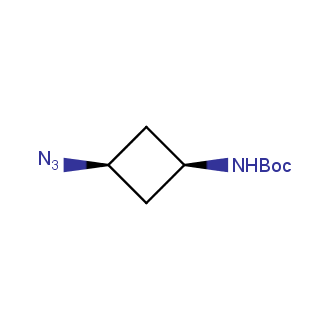
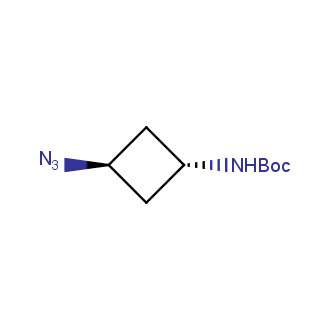
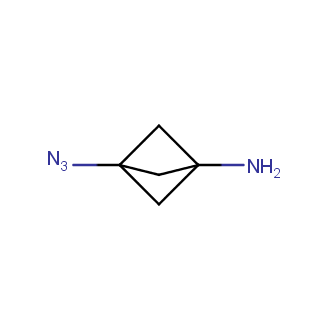
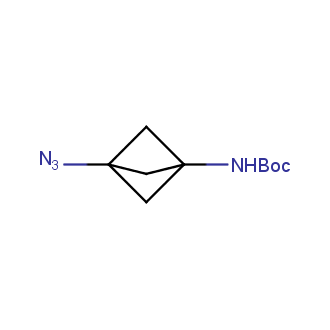
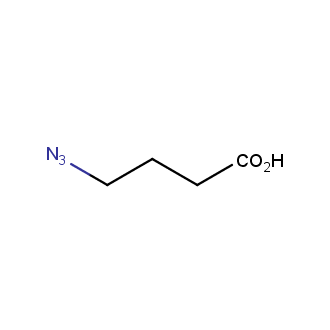
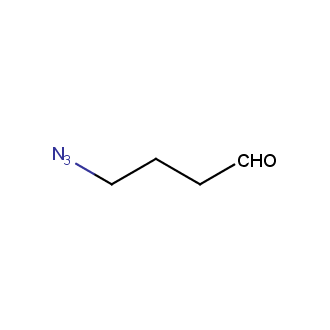
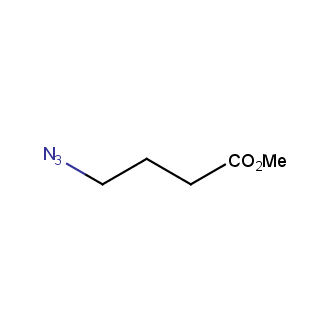
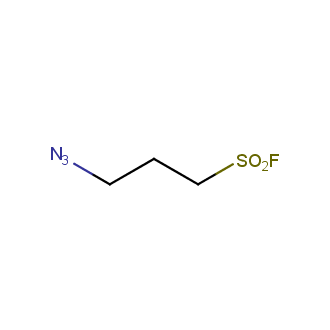
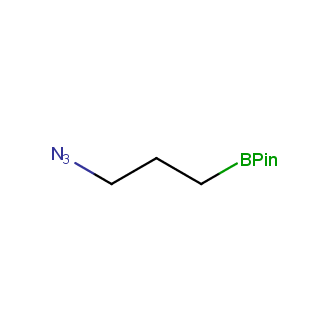
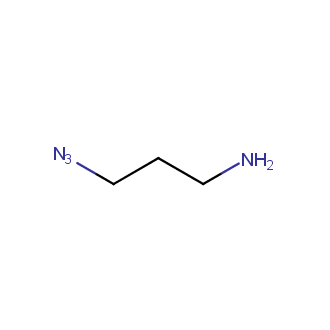
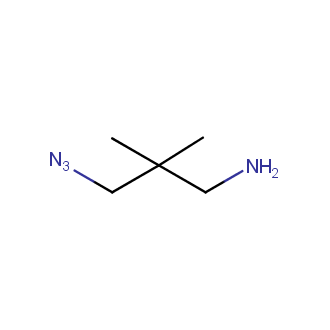
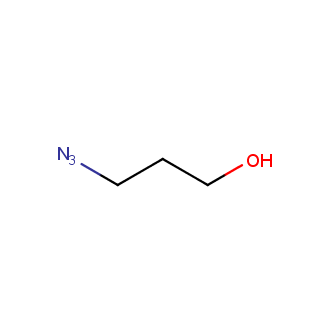
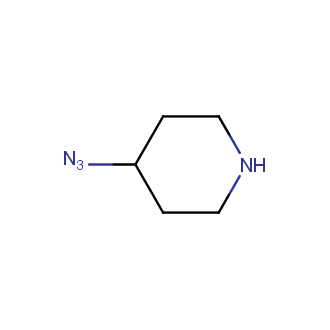
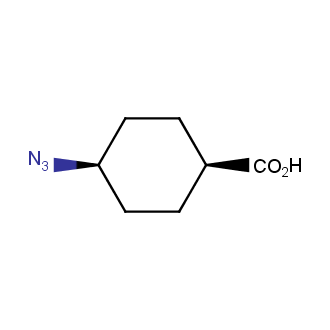
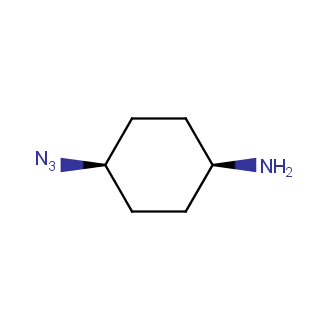
Organic azides became enormously popular for their participation in the Cu(I)-catalyzed Huisgen azide-alkyne 1,3R09;dipolar cycloaddition reaction – “click chemistry”. 1,2,3-triazole function formed by click reaction between an azide and alkyne bears a physicochemical resemblance to the amide bond. Besides, “click chemistry” involves functionalities that can be introduced in small molecules and into specific locations in biomolecules. “Click chemistry” continues to gain popularity and is used in a variety of research fields with significant contributions to the fields of bioconjugation and drug discovery.
Advantages
- Wide in scope.
- Form stable products.
- Give very high yields.
- The presence of the azide-group and a functional group allows the molecule to be modified before or after the click reaction.
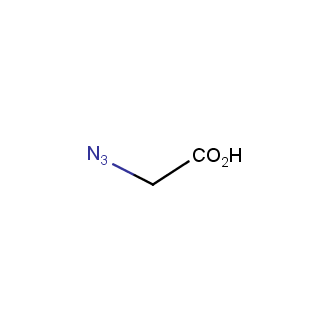
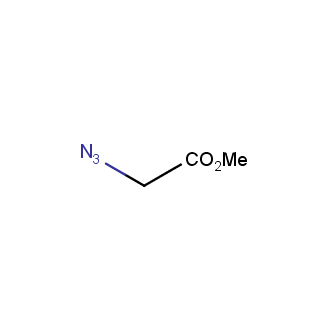
Our offer
>100 different building blocks in multi gram amounts in stock. We also have designed a library azide-containing building blocks for drug discovery programs. These molecules can be synthesized upon request within 4-6 weeks.