CAS 87576-94-1, Cat. No EN300-174337
Reagent for cycloaddition and nucleophilic nitrogen source
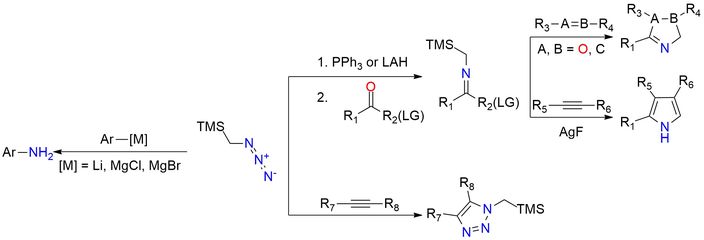
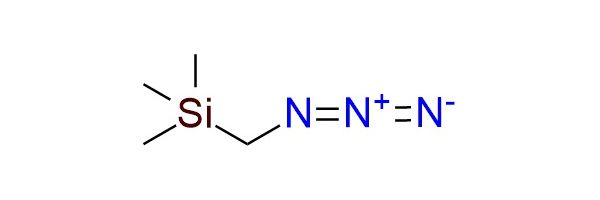
Trimethylsilylmethyl azide (TMSCH₂N₃) is a stable, safe, and versatile azide reagent used as a nucleophilic nitrogen source and as a precursor to reactive 1,3-dipoles1. Unlike methyl azide, TMSCH₂N₃ is non-explosive, thermally stable up to 120 °C, and can be stored for months in a refrigerator. Prepared from chloromethyl-trimethylsilane and NaN₃, it is a colorless, organic-soluble liquid (bp 43 °C/43 mmHg). The reagent is widely used for the amination of aryl Grignard reagents, producing anilines in 70–95% yield via triazene intermediates, and also aminates aryl lithium reagents. TMSCH₂N₃ is an important precursor of azomethine ylides, generated through reduction or Staudinger reaction, which undergo [3+2] cycloadditions with alkenes, alkynes, and carbonyl compounds to form pyrrolines, oxazoles, and other heterocycles. It also participates in cycloadditions with activated alkynes, phosphaalkynes, and isothiocyanates, giving access to diverse 5-membered heterocycles.
Synonyms: (azidomethyl)(trimethyl)silane; azidomethyl-trimethyl-silane; (azidomethyl)trimethyl silane; silane, (azidomethyl)trimethyl-; azidomethyltrimethylsilane; azidomethyl(trimethyl)silane
Selected publication
-
Trimethylsilylmethyl Azide.
Nishiyama, K. Encyclopedia of Reagents for Organic Synthesis 2001. DOI: 10.1002/047084289X.rt316