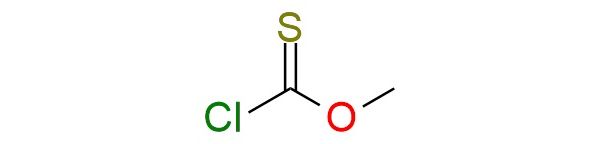
CAS 2812-72-8, Cat. No EN300-303708
Reagent for thionoester synthesis
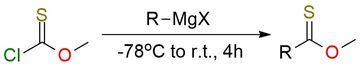
Methyl chlorothioformate is showcased as a practical, scalable electrophile for installing the methyl thionoester motif under transition-metal-free conditions1. With aryl and alkyl Grignard reagents, it is capable of forming many diverse thionoesters. The methodology allows the formation of Grignard’s reagents of aryl bromides in situ. i-PrMgCl·LiCl (turbo-Grignard) improves yields for fluoroaryl partners. The scope of reaction includes almost all substrates, except bearing tertiary amines or protected carboxyls, because they are proven incompatible (e.g., amine dealkylation). The method scales to 20–25 g per run and expands access to thionoesters useful for downstream reductions to ethers, organometallic additions, and dethiofluorination to difluoroethers.
Synonyms: methylsulfanylformyl chloride; formic acid, chlorothio-, O-methyl ester; methoxythiocarbonyl chloride; O-methyl chlorothioformate; O-methyl thiochloroformate
Selected publication
-
Methyl Chlorothioformate as a Convenient Reagent for Thionoester Synthesis.
Pashko, M. O.; Pashkov, K. V.; Granat, D. S.; Yagupolskii, Y. L.; Ryabukhin, S. V.; Volochnyuk, D. M. RSC Adv 2025, 15 (19), 15116–15120. DOI: https://doi.org/10.1039/d5ra01538c