CAS 88738-78-7, Cat. No EN300-298220
Reagent for Still–Gennari olefination
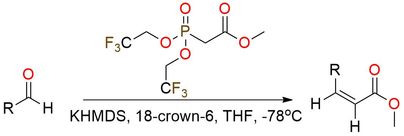
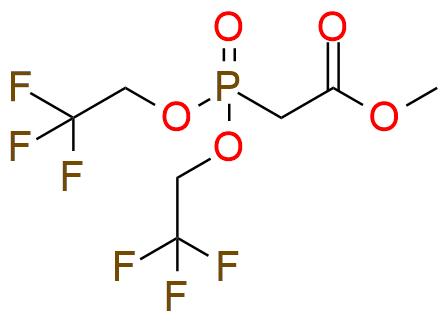
Methyl bis(2,2,2-trifluoroethoxy)phosphinylacetate is used for Still-Gennari modification of the Horner-Wadsworth-Emmons reaction, which selectively yields Z-isomer. The reagent is oil, bench-stable, and has good solubility in THF, DME, and DCM1. The reaction is not overly sensitive to the type of aldehyde used but for successful conversion of product to corresponding α,β-unsaturated esters, the kinetic control of the reaction and strong base is required2. The classical base for the reaction is KHMDS in the pair with 18-crown-6 ether. Usually Still–Gennari olefination doesn’t proceed with ketones, especially with aryl ketones, and is tolerant toward any other functional groups. But it’s important to remember that some ketones can slowly react with the reagent, yielding the side products.
Synonyms: bis(2,2,2-trifluoroethyl) (methyoxycarbonylmethyl)phosphonate
Selected publication
-
Methyl Bis(2,2,2-Trifluoroethoxy)Phosphinylacetate.
DeHoff B.; Roy M.-N. Encyclopedia of Reagents for Organic Synthesis 2012. DOI: 10.1002/047084289X.rm145.pub2
-
Still–Gennari Olefination and Its Applications in Organic Synthesis.
Janicki I.; Kiełbasiński P. Adv Synth Catal 2020, 362 (13), 2552–2596. DOI: 10.1002/adsc.201901591