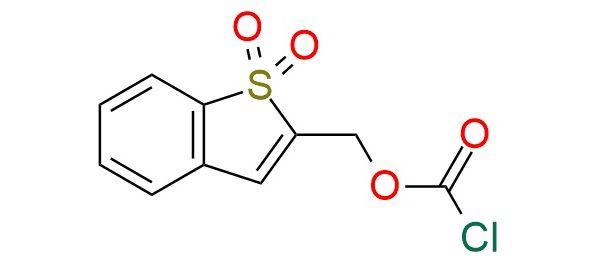
CAS 135204-19-2, Cat. No EN300-7441142
Reagent for amino group protection
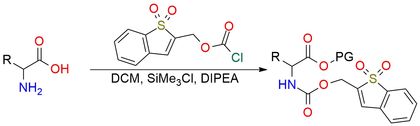
1,1-Dioxobenzo[b]-thiophene-2-yl methyloxycarbonyl chloride (Bsmoc-Cl) is a moisture-sensitive reagent used for amino group protection, particularly the α-amino function of amino acids1. Bsmoc-Cl serves as an alternative to Fmoc in solid-phase and rapid continuous solution peptide synthesis2. Bsmoc-protected amino acids offer advantages such as milder deprotection conditions (lower piperidine concentrations), minimizing base-induced side reactions. The reagent enables selective deprotection due to its distinct Michael-like addition mechanism, allowing orthogonal removal alongside Fmoc groups3. Bsmoc-Cl is stable when dry, soluble in common organic solvents. Hydrolysis occurs readily on silica gel, so it should be handled under anhydrous conditions.
Synonyms: Bsmoc-Cl; 1,1-dioxobenzo[b]thiophen-2-ylmethyl chloroformate; (1,1-dioxidobenzo[b]thiophen-2-yl)methyl carbonochloridate; (1,1-dioxo-1-benzothiophen-2-yl)methyl carbonochloridate; (1,1-dioxo-1lambda6-benzothiophen-2-yl)methyl chloroformate; benzo[b]thiophenesulfone-2-methyloxycarbonyl chloride; benzo[b]thiophenesulfone-2-methyl chloroformate
Selected publications
-
1,1-Dioxobenzo[ b ]-Thiophene-2-Yl Methyloxycarbonyl Chloride.
Carpino, L. A. Encyclopedia of Reagents for Organic Synthesis 2002. DOI: 10.1002/047084289X.rn00086
-
New Family of Base- and Nucleophile-Sensitive Amino-Protecting Groups. A Michael-Acceptor-Based Deblocking Process. Practical Utilization of the 1,1-Dioxobenzo[ b ]Thiophene-2-Ylmethyloxycarbonyl (Bsmoc) Group.
Carpino, L. A.; Philbin, M.; Ismail, M.; Truran, G. A.; Mansour, E. M. E.; Iguchi, S.; Ionescu, D.; El-Faham, A.; Riemer, C.; Warrass, R.; Weiss, M. S. J Am Chem Soc 1997, 119 (41), 9915–9916. DOI: 10.1021/ja9713690
-
The 1,1-Dioxobenzo[ b ]Thiophene-2-Ylmethyloxycarbonyl (Bsmoc) Amino-Protecting Group.
Carpino, L. A.; Ismail, M.; Truran, G. A.; Mansour, E. M. E.; Iguchi, S.; Ionescu, D.; El-Faham, A.; Riemer, C.; Warrass, R. J Org Chem 1999, 64 (12), 4324–4338. DOI: 10.1021/jo982140l