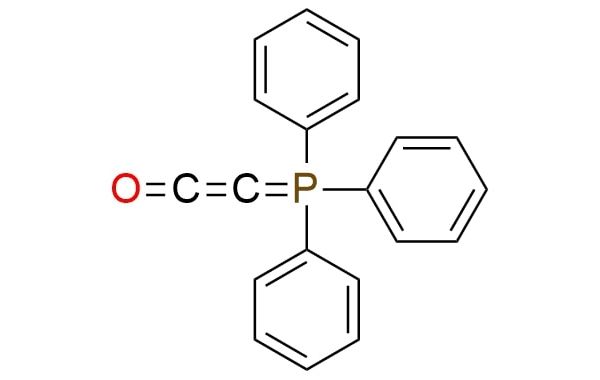
CAS 15596-07-3, Cat. No EN300-7352927
Phosphonium ylide
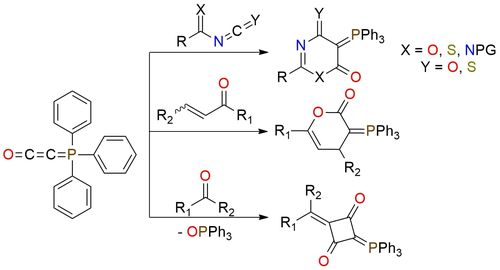
The Bestmann ylide serves as a versatile foundation for synthesizing diverse heterocycles with varying sizes and functionality. This stable, flaky powder is soluble in DCM, dioxane, toluene, benzene, and THF but remains insoluble in diethyl ether1. Diverging from ketenes, this ylide doesn't tend to dimerize; however, it exhibits ketene-like reactivity in the presence of electrophiles1. If the ylide proves a stronger nucleophile than the counter anion, it undergoes a [2 + 2] cycloaddition with a second unreacted ylide molecule, resulting in a 1,3-cyclobutanedione derivative1. Synthesis of heterocycles is possible if the acidic compound contains an additional group capable of cyclization with the initially formed ylide function. This approach has proved to be most useful for the synthesis of five- and six-membered heterocycles, as well as for the preparation of macrocyclic lactones1.
Synonyms: ketenylidenetriphenylphosphorane; 2-(triphenylphosphoranylidene)ethenone; (triphenylphosphoranylidene)ethenone; (triphenylphosphoranylidene)ketene
Selected publication
-
Ketenylidenetriphenylphosphorane.
Bestmann H.; Zimmermann R.; Riou M. Encyclopedia of Reagents for Organic Synthesis 2011. DOI: 10.1002/047084289X.rk005.pub2